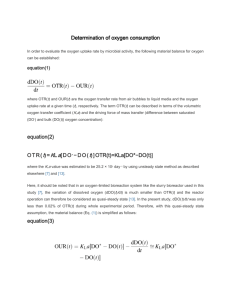
T02 OUR2013
advertisement

Topic 02: Microbial Oxygen Uptake Kinetics • After having investigated principles and methods of quantifying the oxygen transfer OTR (from gas phase to solution): • Now we will investigate the oxygen uptake rate OUR behaviour of bacteria • Leading to combining both kinetics in a typical reactor where often OUR = OTR. This allows easy online interpretation of the whole bioprocess 1 OUR – Variation during batch culture In batch culture OUR changes strongly over time due to increase in biomass (X) depletion of substrate (S). X OUR [Substrate] Time However OUR can be considered constant: • over short time intervals (min) • in continuous culture 2 Very useful tool to study microbes and reactor behaviour OUR – Significance 1. Critical indicator of culture status (respiration rate). 2. Indicator or growth (relationship X* / OUR). 3. Indicator of health, inhibition etc ( if X = constant). 4. Essential for culture optimisation. 5. Should be ideally monitored online. *) X= biomass concentration (e.g. g Dry Weight/L) 3 OUR – Determination 1. Aerate to maximum 2. Stop aeration cL 3. Monitor cL Time (sec) Conclusion: 1. OUR is linear over most cL values 2. A critical D.O. exists 4 OUR – Dependency on DO Maximum rate D.O. (mg/L) about half maximum rate Time (sec) The next slide shows the green and blue part of this curve but as the rate as a function of D.O. 5 OUR – Dependency on DO OUR (mg/L/h) The OUR is mostly independent of D.O. (zero order kinetics) At very low D.O. the OU R is strongly dependent on D.O. (close to 1st order kinetics) D.O. (mg/L) 6 OUR – Dependency on DO Examinable concepts: OUR (mg/L/h) D.O. saturation D.O. limitation First order reaction Zero order reaction Michaelis Menten kinetics Driving Force Equilibrium D.O. (mg/L) 7 Critical DO OUR (mg/L/h) Dependence of OUR on the dissolved oxygen concentration (DO or cL ) 0.5 1 2 DO (mg/L) Conclusions: 1. Typical Michaelis Menten relationship 2. ks at about 0.1 ppm (critical D.O.: 0.2 mg/L) 3. Over most DO concentations 8 Simultaneous OTR and OUR makes the bioreactor more complex • The previous slides have shown OUR without new air input • The next slides consider oxygen transfer rate (OTR) at the same time as oxygen uptake rate (OUR) 9 Simultaneous OUR – OTR Oxygen Steady State Klein T, Schneider K, Heinzle (2012) Biotechnology&Bioengineering 110: 535-542 10 OUR – Indirect online monitoring Steady state: 1. OUR constant 2. OTR constant Air Off 3. DO constant 4. OUR = OTR A dcL/dt = 0 DO (ppm) Air On B dcL/dt = OUR = - QO2.X A B C Time (sec) When dcL/dt = 0 C dcL/dt = OTR - OUR = kLa (cs - cL) - QO2.X → OUR = OTR → OUR = kLa(cs – cL) Conclusion: When kLa is known, steady state OUR can be calculated from the dissolved [oxygen] (D.O.) (cL) 12 OUR – Dependency on DO Feed On DO (ppm) Feed On Feed Off Feed Off Feed Off Time (min) The addition of feed to a starving culture of microbes results in an instantaneous increase of OUR, which Causes a drop in the D.O. 14 OUR – calculation from in situ DO monitoring 1. Calculation of OUR from kLa and cL Given: • Reactor with airflow that gives a known kLa of . kLa = 20 h-1 • Due to bacterial OUR a steady state DO establishes at 2 mg/L • OUR = kLa * (cS-cL) kLa = 20 h-1, cL = 2 mg/L, cS= 8 mg/L OUR = ? OUR = 20 h-1 x 6 mg/L = 120 mg/L/h Conclusion: OUR can be determined immediately Online OUR monitoring is possible (online respirometry) Useful for Degradability tests, toxicity tests, process optimisation 15 OUR – calculation from in situ DO monitoring 2. Determination of kLa in situ (dynamic method) Since under steady state: OTR = OUR kLa = OUR (cs – cL) 16 OUR – applications of online DO monitoring 3. Calculation of new OUR from old OUR and cL Original OUR = 120 mg/L/h at cL of 4 mg/L After further growth DO lowered to 2 mg/L What is the new OUR? kLa = OUR (cS – cL) = 120 mg/L/h 4 mg/L = 30 h-1 OUR = kLa * (cS – cL) = 30 h-1 * (8 mg/L – 2 mg/L) = 180 mg/L 17 OUR – applications of online DO monitoring 4. Calculation of new kLa from old kLa and cL Original kLa = 30 h-1 at cL of 2 mg/L After increasing airflow the new cL was 5 mg/L What is the new kLa? OUR = kLa * (cS – cL) = 30 h-1 * (8 mg/L – 2 mg/L) = 180 mg/L/h OUR new kLa = (cS – cL) = 180 mg/L/h 3 mg/L = 60 h-1 18 OUR- Comparison of Methods for kLa determination 1. Static Gassing Out Method (N2) De-oxygenate solution, monitor DO increase over time. Determine kLa (a) graphically or (b) mathematically 2. Sulfite Method Sulfite reactos spontaneously with D.O (in the presence of a cobalt catalyst – as also used in our lab session) Titration of sulfite consumption during oxygenation trial - indirect measurement + no oxygen probe required + allows direct monitoring of standard OTR standard OTR = kLa . cs 3. Dynamic Method (in situ kLa) + measured real in situ value considering changes of medium such as viscosity, particles, surface tension ... - depends upon known OUR + only slight process interuption necessary + only works when DO >> critical DO 19 OUR- Comparison of Methods for kLa determination 4. Oxygen Balance Method = Direct Monitoring OTR = specific air flow . ([O2]in - [O2]out ) mg/L.h L(g)/L(l).h mg/L(g) Measures the oxygen concentration in the exit air of the reactor + integrates over the whole reactor volume + not affected by fine air bubbles (which still transfer some oxygen for a while even after stopping of air flow) + no response lag by oxygen probe - Longer response time to step changes - Lower precision of oxygen readings in air than in solution 20 OUR – indirect respiration activity monitoring There is very useful information in the OUR response of microbial cultures to the addition of substrates or inhibitors D.O. (mg/L) Add feed Time Effect of minute feed addition on D.O. profile of aerated starving microbial culture 21 OTR OUR D.O D.O. (mg/L) cS D.O cS Add feed Time Effect of minute feed addition on D.O. profile of aerated starving microbial culture 22 OUR – indirect respiration activity monitoring OUR (mg/L/h) D.O. (mg/L) Add feed Time Effect of minute feed addition on D.O. profile of aerated starving microbial culture 23 OUR – indirect respiration activity monitoring 40 OUR (mg/L/h) 20 20 mg/L/h * 0.1h = 2 mg/L 6 12 Time (min) 24 OUR response to feed spike by starving microbial culture. Numerical integration (counting squares) allows to determine the amount of oxygen used due to the feed spike addition. 24 Lecture overview L 4-6 Lab1 (computerlab): Intro to CBLA use, oxygen solubility, show bioprosim, download material, use memory sticks, Henry’s law, temperature effect on oxygen solubility. Use of spreadsheets for data processing Lecture 4: In situ method of determining kLa sulfite method of determining kLa Lecture 5: Online OUR monitoring as a key bioprocess monitoring tool. Saturation behaviour of OUR. Critical DO. Respirometric testing of substrates and inhibitors. Numeric integration of rate data