16.4-Colligative calculations and Molality
advertisement
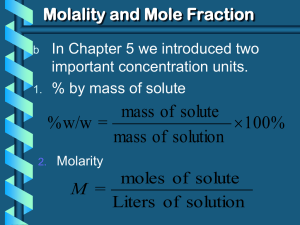
Calculations & Colligative Properties Chapter 16.4 Learning Objectives • Know the difference between molality and molarity • Be able to calculate molality • Can use molality to calculate freezing point depression or boiling point elevation • Understand how ionic compounds affect colligative properties • Can calculate mole fractions Molarity vs Molality Molarity (M) M = moles of solute liters of solution Molality (m) m = moles of solute mass of solvent (kg) Molarity vs Molality so 3.2 M means we have 3.2 moles in 1 liter of solution so 3.2 m means we have 3.2 moles in 1 kg of solvent Why do we need to know molality? Colligative properties ….. We use molality to make calculations for boiling point elevation or freezing point depression Boiling Point Elevation DTb = Kb Change in boiling point x m x Molality of solution Molal boiling point constant (0.51 oC/m for water) i Van’t Hoff factor Freezing Point Depression DTf = Kf Change in freezing point x m x Molality of solution Molal freezing point constant (-1.86 oC/m for water) i Van’t Hoff factor Important note about these calculations! The calculations change depending on whether you have a nonelectrolyte or electrolyte solution Why? 1 C12H22O11 (s) 1 C12H22O11 (aq) (i =1) Sugar does not dissociate into ions! 1 Ba(NO3)2 (s) 1 Ba2+(aq) + 2 NO3-1(aq) (i =3) Barium nitrate will lower the freezing point of its solvent three times as much as sugar of the same molality Sample Calculation: Freezing Point Depression: Electrolyte What is the freezing point depression of water in a solution of 62.5 g of barium nitrate, Ba(NO3)2, in 800. g of water? DTf = Kf •m•i Let’s calculate m first m = moles of Ba(NO3)2 / kg solvent 800. g = 0.800 kg 137.3(1) 14(2) 16(6)_____ 261.3g/mol m = 0.239 moles / 0.800 kg water 62.5 g 261.3 g/mol m = 0.299 mol/kg DTf = (-1.86 oC/m)(0.299 m)(3) Van’t Hoff factor (i) DTf = -1.70 oC Mole Fraction • Mole fractions have no units nA XA n A nB nB XB n A nB XA + XB = 1 Mole Fraction Calculation A solution has 1.43 moles of vanadium oxide in 3.45 moles of chloroform. What is the mole fraction of each component in the solution? nA XA n A nB nB XB n A nB XA = 1.43 / 1.43 + 3.45 XA = 0.293 XA = 3.45 / 1.43 + 3.45 XA = 0.707 0.293 + 0.707 = 1