The Number Distribution
advertisement

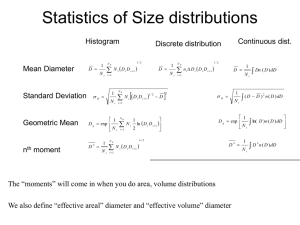
Size Distributions • Many processes and properties depend on particle size – – – – – • Fall velocity Brownian diffusion rate CCN activity Light scattering and absorption Others There are a number of quantitative ways to represent the size distribution – – – – – – – Histogram Number Distribution Number Distribution function Volume, area, mass distributions Cumulative distributions Statistics of size distributions: Median, mode, averages, moments… Others • We will review a number of these next • Our primary goal is to explain the Number Distribution Function, which is the most widely used Size Distributions The Histogram Simplest form of distribution – Very instrument-based Lots of structure at small sizes Few particles at largest sizes Di –Di+1 Ni i 1, 2, . . . N B NB = Number of size bins Di = Lower-bound particle diameter for bin i Di+1 = Upper-bound particle diameter for bin i Ni = concentration of particles in bin i (cm-3) Size Distributions Cumulative Properties from Histogram NB Total Concentration Nt Ni i 1 NB Total Surface Area Total Volume Di –Di+1 i 1 NB Vt i 1 Ni i = density of aerosol substance in bin i St Di Di 1 Ni Total Mass Note: We don’t have an “average” diameter for the bin – only the bin boundaries. Above I use the geometric mean. Sometimes it makes sense to estimate where the particles are within the bin based on the concentrations of neighboring bins, and then calculate the effective mean diameter. D D 6 i NB i 1 6 Mt 3/ 2 i 1 Ni i Di Di 1 N i 1/ N N Ag Ai i 1 1 N exp ln Ai N i 1 3/ 2 Size Distributions Cumulative Distributions i 1 Cum. Concentration N ( Di ) N j j 1 i 1 Cum. Surface Area S ( Di ) D j D j 1 N j j 1 i 1 Cum. Volume i Di –Di+1 Ni N ( Di 1 ) N j V ( Di ) j 1 j 1 Cum. Mass D D 6 i 1 j 1 6 M ( Di ) 3/ 2 j j 1 Nj j D j D j 1 N j 3/ 2 A Cumulative distribution gives the concentration (or some other property) of particles smaller than diameter Di • • • • • Cumulative values are properties at bin boundaries, not bin centers! They are monotonically increasing in size N(DNB+1) = Nt Ni N ( Di 1 ) N ( Di ) Different instruments should report the same function, just sampled differently Size Distributions The Number Distribution More uniform way to present instrument data i Di –Di+1 Ni N ( Di 1 ) N j j 0 ni = aerosol number distribution for bin i DDi = Di+1–Di is the bin width Ni = niDDi ni = has units of ni (cm-3 mm-1) i N ( Di 1 ) n j DD j j 0 Area under the curve = total aerosol concentration, N Size Distributions The Number Distribution Histogram Number distribution Small bin width at small sizes leads to amplification of concentrations here relative to histogram Instruments with different Di would produce very different histograms, but similar number distributions Size Distributions The Log Number Distribution ni Aerosol distributions span orders of magnitude in size, and are often best shown as a function of log-diameter. Now, the area under curve is NOT equal to total concentration. To remedy this, we can create a log number distribution (not shown above) ni0 Ni Ni D log Di logDi 1 / Di Size Distributions The Number Distribution Function Distributions are often represented in models or analytically, as continuous functions of diameter. This is as if we had an number distribution with perfectly precise resolution n( D p ) lim Di1 Di Ni Di 1 Di This looks a lot like the definition of the derivative. If we use the cumulative distribution, we get… ni The log-diameter distribution is the derivative of the cumulative distribution with log of diameter n0 ( Dp ) dN ( D) dN( D) 2.303D d log D dD n( D ) N Di 1 N Di dN( D) lim Di1 Di dD Di 1 Di We think of the number distribution function as the derivative with diameter of the cumulative distribution When n(D) is plotted vs. D (NOT logD), then the area under the curve = total concentration Size Distributions Other Distribution Functions n( D ) Number Distribution dN dD Surface Distribution nS ( D ) dS D 2 n( D) dD Volume Distribution nV ( D) dV 3 D n( D ) dD 6 Mass Distribution nM ( D) dM ( D ) D 3 n( D ) dD 6 Aerosol distributions span orders of magnitude in size, and are often best shown as a function of log-diameter. We must use the identity nN0 (logD) 2.303DnN ( D) This lowers the power of Dn in the functions above. Note the “shifting of the peaks” from number area volume Statistics of Size distributions Histogram Mean Diameter Standard Deviation 1 D Nt Discrete distribution 1/ 2 NB N D D i 1 D 1 Nt Geometric Mean 1 Dg exp Nt nth moment 1 Dn Nt NB i 1 D Nt i 1 i N D D NB i 1 1/ 2 i i 1 i D 1/ 2 NB n DD D D i 1 Continuous dist. i i i D i 1 2 1 N ln D D i i i 1 2 i 1 NB n/2 N i Di Di 1 i 1 The “moments” will come in when you do area, volume distributions D 1 Dn( D)dD Nt 1 ( D D ) 2 n( D)dD Nt 1 Dg exp ln(D)n( D)dD Nt Dn 1 D n n( D)dD Nt More Statistics In-class… • Power-law distributions • Log-normal distributions • Properties of each