Electromagnetic Radiation
advertisement

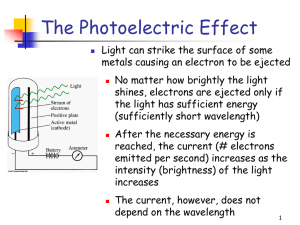
(Electron Configurations) Electromagnetic Radiation-form of energy that exhibits wave-like behavior as it travels through space. Electromagnetic Spectrum-ordered arrangement by wavelength or frequency for all forms of electromagnetic radiation. Wavelength-lambda (λ) The distance between corresponding points on adjacent waves. Units: m, nm, cm, or Å Frequency-nu (ν) The number of waves passing a given point in a definite amount of time. Units: hertz (Hz) or cycles/sec = 1/sec = sec-1 When an electric field changes, so does the magnetic field. The changing magnetic field causes the electric field to change. When one field vibrates—so does the other. RESULT-An electromagnetic wave. Waves or Particles Electromagnetic radiation has properties of waves but also can be thought of as a stream of particles. Example: Light Light as a wave: Light behaves as a transverse wave which we can filter using polarized lenses. Light as particles (photons) When directed at a substance light can knock electrons off of a substance (Photoelectric effect) c = λ∙ν λ = wavelength (m) ν = frequency (Hz) c = speed of light= 3.0 x 108 m/sec (constant) λ and ν are _______________ related. Truck-mounted helium-neon laser produces red light whose wavelength (λ ) is 633 nanometers. Determine the frequency (v). *Remember that c=3.0x108m/s. *Use the formula v= c λ c =3.0x108 m/s c= λ . v v=c / λ λ = 633nm= 6.33x10-7m v = 3.0x108 m/s = 0.47x 1015s-1 = 4.7x1014 s-1 6.33x10-7m Frequency = 4.7x1014 Hz (cycles per second) EX: Find the frequency of a photon with a wavelength of 434 nm. GIVEN: WORK: =c =? = 434 nm = 4.34 10-7 m = 3.00 108 m/s -7 m 8 4.34 10 c = 3.00 10 m/s = 6.91 1014 Hz 2 problems that could not be explained if light only acted as a wave. 1.) Emission of Light by Hot bodies: Characteristic color given off as bodies are heated: red yellow white If light were a wave, energy would be given off continually in the infrared (IR) region of the spectrum. 2.) Absorption of Light by Matter = Photoelectric Effect Light can only cause electrons to be ejected from a metallic surface if that light is at least a minimum threshold frequency . The intensity is not important. If light were only a wave intensity would be the determining factor, not the frequency! When an object loses energy, it doesn’t happen continuously but in small packages called “quanta”. “Quantum”-a definite amount of energy either lost or gained by an atom. “Photon”-a quantum of light or a particle of radiation. Calculate the frequency for the yelloworange light of sodium. Calculate the frequency for violet light. Calculate the frequency for the yelloworange light of sodium. Calculate the frequency for violet light. E = h∙ν E = energy (joule) h = Planck’s constant = 6.63 x 10-34 j∙sec ν = frequency (Hz) E and ν are ______________ related. Calculate the energy for the yellow-orange light for sodium. Calculate the energy for the violet light. Excited State: Higher energy state than the atom normally exists in. Ground State: Lowest energy state “happy state” Line Spectrum: Discrete wavelengths of light emitted. 2 Types: 1.) Emission Spectrum: All wavelengths of light emitted by an atom. 2.) Absorption Spectrum: All wavelengths of light that are not absorbed by an atom. This is a continuous spectrum with wavelengths removed that are absorbed by the atom. These are shown as black lines for absorbed light. Continuous Spectrum: All wavelengths of a region of the spectrum are represented (i.e. visible light) Hydrogen’s spectrum can be explained with the wave-particle theory of light. Niel’s Bohr (1913) 1.) The electron travels in orbits (energy levels) around the nucleus. 2.) The orbits closest to the nucleus are lowest in energy, those further out are higher in energy. 3.) When energy is absorbed by the atom, the electron moves into a higher energy orbit. This energy is released when the electron falls back to a lower energy orbit. A photon of light is emitted. Lyman Series-electrons falling to the 1st orbit, these are highest energy, _____ region. Balmer Series- electrons falling to the 2nd orbit, intermediate energy, _______ region. Paschen Series-electrons falling to the 3rd orbit, smallest energy, ______ region. En = (-RH) 1/n2 En = energy of an electron in an allowed orbit (n=1, n=2, n=3, etc.) n = principal quantum number (1-7) RH = Rydberg constant (2.18 x 10-18 J) When an electron jumps between energy levels: ΔE =Ef – Ei By substitution: ΔE = hν = RH(1/ni2 - 1/nf2) When nf > ni then ΔE = (+) When nf < ni then ΔE = (-) DeBroglie (1924)-Wave properties of the electron was observed from the diffraction pattern created by a stream of electrons. Schrodinger (1926)-Developed an equation that correctly accounts for the wave property of the electron and all spectra of atoms. (very complex) Rather than orbits we refer to orbitals. These are 3-dimensional regions of space where there is a high probability of locating the electron. Heisenberg Uncertainty Principle-it is not possible to know the exact location and momentum (speed) of an electron at the same time. Quantum Numbers-4 numbers that are used to identify the highest probability location for the electron. 1.) Principal Quantum Number (n) States the main energy level of the electron and also identifies the number of sublevels that are possible. n=1, n=2, n=3, etc. to n=7 2.) Orbital Quantum Number Identifies the shape of the orbital s (2 electrons) P (6 electrons) d (10 electrons) f (14 electrons) sphere dumbbell 1 orbital 3 orbitals 4-4 leaf clovers & 1-dumbbell w/doughnut5 orbitals very complex 7 orbitals 3.) Magnetic Quantum Number Identifies the orientation in space (x, y, z) s 1 orientation p 3 orientations d 5 orientations f 7 orientations 4.) Spin Quantum Number States the spin of the electron. Each orbital can hold at most 2 electrons with opposite spin. 1.) Principal Quantum Number (n) States the main energy level of the electron and also identifies the number of sublevels that are possible. n=1, n=2, n=3, etc. to n=7 2.) Azimuthal Quantum Number (l) Values from 0 to n-1 Identifies the shape of the orbital l=0 l=1 l=2 l=3 s p d f sphere dumbbell 1 orbital 3 orbitals 4-4 leaf clovers & 1-dumbbell w/doughnut5 orbitals very complex 7 orbitals 3.) Magnetic Quantum Number (ml) Values from –l l States the orientation in space (x, y, z) ml = 0 ml = -1, 0, +1 ml = -2,-1,0,+1,+2 ml = -3,-2,-1,0,+1+2,+3 s p d f only 1 orientation 3 orientations 5 orientations 7 orientations 4.) Spin Quantum Number (ms) Values of +1/2 to -1/2 States the spin of the electron. Each orbital can hold at most 2 electrons with opposite spin.