Product Training 2007 Elemental and Process Analysis
advertisement

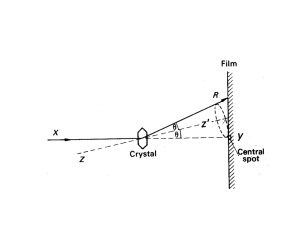
Basic Crystallography Part 1 Theory and Practice of X-ray Crystal Structure Determination Charles Campana, Ph.D. Senior Applications Scientist Bruker AXS Course Overview Basic Crystallography – Part 1 Introduction: Crystals and Crystallography Crystal Lattices and Unit Cells Generation and Properties of X-rays Bragg's Law and Reciprocal Space X-ray Diffraction Patterns from Crystals Basic Crystallography – Part 2 Review of Part 1 Selection and Mounting of Samples Unit Cell Determination Intensity Data Collection Data Reduction Structure Solution and Refinement Analysis and Interpretation of Results Introduction to Crystallography What are Crystals? 4 Examples of Crystals Examples of Protein Crystals Growing Crystals Kirsten Böttcher and Thomas Pape Crystal Systems and Crystal Lattices What are Crystals? A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly, repeating pattern extending in all three spatial dimensions. 9 Foundations of Crystallography Crystallography is the study of crystals. Scientists who specialize in the study of crystals are called crystallographers. Early studies of crystals were carried out by mineralogists who studied the symmetries and shapes (morphology) of naturally-occurring mineral specimens. This led to the correct idea that crystals are regular threedimensional arrays (Bravais lattices) of atoms and molecules; a single unit cell is repeated indefinitely along three principal directions that are not necessarily perpendicular. The Unit Cell Concept Ralph Krätzner Unit Cell Description in terms of Lattice Parameters a ,b, and c define the edge lengths and are referred to as the crystallographic axes. c a b , , and give the angles between these axes. Lattice parameters dimensions of the unit cell. Choice of the Unit Cell Choice of the Unit Cell A B A B C D No symmetry - many possible unit cells. A primitive cell with angles close to 90º (C or D) is preferable. C The conventional C-centered cell (C) has 90º angles, but one of the primitive cells (B) has two equal sides. 7 Crystal Systems - Metric Constraints Triclinic - none Monoclinic - = = 90, 90 Orthorhombic - = = = 90 Tetragonal - = = = 90, a = b Cubic - = = = 90, a = b = c Trigonal - = = 90, = 120, a = b (hexagonal setting) or = = , a = b = c (rhombohedral setting) Hexagonal - = = 90, = 120, a = b Bravais Lattices Within each crystal system, different types of centering produce a total of 14 different lattices. P – Simple I – Body-centered F – Face-centered B – Base-centered (A, B, or C-centered) All crystalline materials can have their crystal structure described by one of these Bravais lattices. Bravais Lattices Cullity, B.D. and Stock, S.R., 2001, Elements of X-Ray Diffraction, 3rd Ed., Addison-Wesley Bravais Lattices Cullity, B.D. and Stock, S.R., 2001, Elements of X-Ray Diffraction, 3rd Ed., Addison-Wesley Bravais Lattices Bravais Lattices Crystal Families, Crystal Systems, and Lattice Systems Generation of and Properties of X-rays A New Kind of Rays Wilhelm Conrad Röntgen German physicist who produced and detected Röntgen rays, or X-rays, in 1895. He determined that these rays were invisible, traveled in a straight line, and affected photographic film like visible light, but they were much more penetrating. Properties of X-Rays Electromagnetic radiation (l = 0.01 nm – 10 nm) Wavelengths typical for XRD applications: 0.05 nm to 0.25 nm or 0.5 to 2.5 Å 1 nm = 10-9 meters = 10 Å E = ħc / l Generation of Bremsstrahlung Radiation Electron (slowed down and changed direction) nucleus Fast incident electron electrons Atom of the anode material X-ray “Braking” radiation. Electron deceleration releases radiation across a spectrum of wavelengths. Generation of Characteristic Radiation Photoelectron M Emission K L K Electron L K Incoming electron knocks out an electron from the inner shell of an atom. Designation K,L,M correspond to shells with a different principal quantum number. Generation of Characteristic Radiation Not every electron in each of these shells has the same energy. The shells must be further divided. K-shell vacancy can be filled by electrons from 2 orbitals in the L shell, for example. Bohr`s model The electron transmission and the characteristic radiation emitted is given a further numerical subscript. Generation of Characteristic Radiation Energy levels (schematic) of the electrons M Intensity ratios K K K = L K K K K K Emission Spectrum of an X-Ray Tube Emission Spectrum of an X-Ray Tube: Close-up of K Cullity, B.D. and Stock, S.R., 2001, Elements of X-Ray Diffraction, 3rd Ed., Addison-Wesley Sealed X-ray Tube Cross Section Cullity, B.D. and Stock, S.R., 2001, Elements of X-Ray Diffraction, 3rd Ed., Addison-Wesley Sealed tube Cathode / Anode Beryllium windows Water cooled Characteristic Radiation for Common X-ray Tube Anodes Anode K1 (100%) K2 (50%) K (20%) Cu 1.54060 Å 1.54439 Å 1.39222 Å Mo 0.70930 Å 0.71359 Å 0.63229 Å Modern Sealed X-ray Tube Tube made from ceramic Beryllium window is visible. Anode type and focus type are labeled. Sealed X-ray Tube Focus Types: Line and Point Target Take-off angle The X-ray beam’s cross section at a small take-off angle can be a line shape or a spot, depending on the tube’s orientation. Filament Spot Target Line The take-off angle is the targetto-beam angle, and the best choice in terms of shape and intensity is usually ~6°. A focal spot size of 0.4 × 12 mm: 0.04 × 12 mm (line) 0.4 × 1.2 mm (spot) Interaction of X-rays with Matter Interactions with Matter d incoherent scattering lCo (Compton-Scattering) wavelength lPr intensity Io coherent scattering lPr (Bragg-scattering) absorption Beer´s law I = I0*e-µd fluorescence l> lPr photoelectrons Coherent Scattering Incoming X-rays are electromagnetic waves that exert a force on atomic electrons. e- The electrons will begin to oscillate at the same frequency and emit radiation in all directions. Constructive and Deconstructive Interference 1.0 0.8 0.6 1.0 0.2 0.8 0.0 -0.2 0.6 -0.4 = -0.6 -0.8 -1.0 -1.2 -1.4 1.0 0 100 200 300 400 500 600 700 800 900 1000 X Axis Title 0.8 0.6 Y Axis Title 0.4 0.2 0.4 Y Axis Title Y Axis Title 0.4 0.2 0.0 -0.2 -0.4 -0.6 0.0 -0.2 -0.8 -0.4 -0.6 -1.0 -0.8 -1.0 -1.2 -1.2 -1.4 -1.4 0 100 200 300 400 500 600 700 800 900 1000 X Axis Title 0 100 200 300 400 500 600 X Axis Title 1.0 0.8 0.6 0.2 0.0 -0.2 -0.4 -0.6 -0.8 = -1.0 -1.2 -1.4 0 100 200 300 400 500 600 700 800 900 1000 X Axis Title 1.0 0.8 0.6 0.4 Y Axis Title Y Axis Title 0.4 0.2 0.0 -0.2 -0.4 -0.6 -0.8 -1.0 -1.2 -1.4 0 100 200 300 400 500 600 X Axis Title 700 800 900 1000 700 800 900 1000 Coherent Scattering by an Atom Coherent scattering by an atom is the sum of this scattering by all of the electrons. 2q Electrons are at different positions in space, so coherent scattering from each generally has different phase relationships. At higher scattering angles, the sum of the coherent scattering is less. Atomic Scattering Factor Scattering factor is used as an indication of the strength of scattering of an atom in particular direction. Scattering is a maximum in the forward scattering direction and decreases with scattering angle. f= Amplitude of wave scattered by atom Amplitude of wave scattered by one electron X-ray Diffraction by Crystals Diffraction of X-rays by Crystals The science of X-ray crystallography originated in 1912 with the discovery by Max von Laue that crystals diffract X-rays. Von Laue was a German physicist who won the Nobel Prize in Physics in 1914 for his discovery of the diffraction of X-rays by crystals. Max Theodor Felix von Laue (1879 – 1960) X-ray Diffraction Pattern from a Single-crystal Sample Rotation Photograph Diffraction of X-rays by Crystals After Von Laue's pioneering research, the field developed rapidly, most notably by physicists William Lawrence Bragg and his father William Henry Bragg. In 1912-1913, the younger Bragg developed Bragg's law, which connects the observed scattering with reflections from evenly-spaced planes within the crystal. William Henry Bragg William Lawrence Bragg Bragg’s Law X-rays scattering coherently from 2 of the parallel planes separated by a distance d. Incident angle and reflected (diffracted angle) are given by q. Bragg’s Law l 2 = d sin q The condition for constructive interference is that the path difference leads to an integer number of wavelengths. Bragg condition concerted constructive interference from periodically-arranged scatterers. This occurs ONLY for a very specific geometric condition. nl = 2d sin q Bragg’s Law nl = 2d sin(q) q q d We can think of diffraction as reflection at sets of planes running through the crystal. Only at certain angles 2θ are the waves diffracted from different planes a whole number of wavelengths apart (i.e., in phase). At other angles, the waves reflected from different planes are out of phase and cancel one another out. Reflection Indices z These planes must intersect the cell edges rationally, otherwise the diffraction from the different unit cells would interfere destructively. y x We can index them by the number of times h, k and l that they cut each edge. The same h, k and l values are used to index the X-ray reflections from the planes. Planes 3 -1 2 (or -3 1 -2) Examples of Diffracting Planes and their Miller Indices Method for identifying diffracting planes in a crystal system. c A plane is identified by indices (hkl) called Miller indices, that are the reciprocals of the fractional intercepts that the plane makes with the crystallographic axes (abc). b a Diffraction Patterns Two successive CCD detector images with a crystal rotation of one degree per image: For each X-ray reflection (black dot), indices h,k,l can be assigned and an intensity I = F 2 measured Reciprocal Space The immediate result of the X-ray diffraction experiment is a list of X-ray reflections hkl and their intensities I. We can arrange the reflections on a 3D grid based on their h, k and l values. The smallest repeat unit of this reciprocal lattice is known as the reciprocal unit cell; the lengths of the edges of this cell are inversely related to the dimensions of the real-space unit cell. This concept is known as reciprocal space; it emphasizes the inverse relationship between the diffracted intensities and real space. The Crystallographic Phase Problem The Crystallographic Phase Problem In order to calculate an electron density map, we require both the intensities I = F 2 and the phases of the reflections hkl. The information content of the phases is appreciably greater than that of the intensities. Unfortunately, it is almost impossible to measure the phases experimentally! This is known as the crystallographic phase problem and would appear to be unsolvable! Crystal Structure Solution by Direct Methods Early crystal structures were limited to small, centrosymmetric structures with ‘heavy’ atoms. These were solved by a vector (Patterson) method. The development of ‘direct methods’ of phase determination made it possible to solve non-centrosymmetric structures on ‘light atom’ compounds Rapid Growth in Number of Structures in Cambridge Structural Database The Structure Factor F and Electron Density Fhkl = ∫ V xyz exp[+2i(hx+ky+lz)]dV xyz = (1/V) hkl Fhkl exp[-2i(hx+ky+lz)] F and are inversely related by these Fourier transformations. Note that is real and positive, but F is a complex number: in order to calculate the electron density from the diffracted intensities I = F2, we need the PHASE () of F. Unfortunately, it is almost impossible to measure directly! Real Space and Reciprocal Space Real Space Reciprocal Space Unit Cell (a, b, c, , , ) Electron Density, (x, y, z) Atomic Coordinates – x, y, z Thermal Parameters – Bij Bond Lengths (A) Bond Angles (°) Crystal Faces Diffraction Pattern Reflections Integrated Intensities – I(h,k,l) Structure Factors – F(h,k,l) Phase – (h,k,l) X-Ray Diffraction X-ray beam l 1Å (0.1 nm) ~ (0.2mm)3 crystal ~1013 unit cells, each ~ (100Å)3 Diffraction pattern on a CCD detector Summary of Part 1 Introduction to crystals and crystallography Definitions of unit cells, crystal systems, Bravais lattices Introduction to concepts of reciprocal space, Fourier transforms, d-spacing, Miller indices Bragg’s Law – geometric conditions for relating the concerted coherent scattering of monochromatic X-rays by diffracting planes of a crystal to its d-spacing. Part 2 – We will use these concepts and apply them in a practical way to demonstrate how an X-ray crystal structure is carried out.