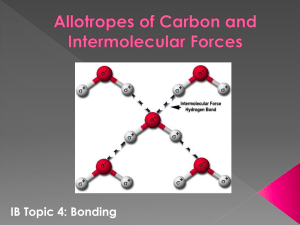
here
advertisement
Tutorial 2 GEM2507 Physical Question from Everyday Life Odor and Photosynthesis Q1.1 Estimate the volume one single air molecule occupies(on average) in the lecture room (let us assume the temperature is 24 oC). Q1.2 From an independent estimate of the volume occupied by a single air molecule in the lecture room by using the ideal gas law. P 1Atm 1.013x105 Pa, T 240 C 297K PV nRT 1 1 Volume of = nRT (1m ol)(8.314Jm ol K )(297K ) 2 3 1 mole of 2 . 44 x 10 m 5 P 1.013x10 Pa air Volume of 1 molecule of air Volume of 1 mol of air = Number of molecules in 1 mol of air 2.44x102 26 3 20 3 4 . 053 x 10 m 4 . 053 x 10 cm 6.02x1023 Q1.3 Deduce an estimate for radius of an air molecule in the lecture room. The radius of an air molecule can be deduced from the density of water Since the density of water is 1000 times the density of air, the volume of space occupied by the air molecule must be 1000 times larger than the volume of one water molecules. Assuming that the water molecules are closely spaced, we obtain 4/3 π r3 = 4.053x 10-20 cm3 /1000 R = 3.04 x 10-8 cm Q1.4 Estimate the average distance between air molecules in the lecture room. Q1.5 Compute the mean free path of the air molecules in the lecture room air molecule Assumption: the air molecules occupy the room uniformly Volume occupied by 1 air molecule d d l d Mean free path l 1 2 4 2nr 4 2 1 1 10 3 . 04 x 10 4.05x1026 2.47x108 m Number of molecules per unit volume = 1/(4.05x10-20 cm3) Q1.6 Use the Avogadro’s number and the definition of the mole to determine the mass of the oxygen molecule Mass of one oxygen molecule = mass of one mole of oxygen/ Avogadro’s number = 32 x 10-3 kg/6.02x1023 = 5.32 x 10-26 kg Q1.7 Repeat the previous question for the nitrogen molecule and then estimate the mean mass of an air molecule Mass of one nitrogen molecule = mass of one mole of nitrogen/ Avogadro’s number = 28 x 10-3 kg/6.02x1023 = 4.65 x 10-26 kg Mean mass of one air molecule = (0.2 x 32 + 0.8 x 28) x 10-3 kg/ 6.02 x 1023 =4.78 x 10-26 kg Q1.8 Estimate the average velocity of an air molecule in the lecture room. ½ mv2 = 3/2 kbT ½ (4.78 x 10-26) v2 = 3/2 (1.38x10-23) x 298 v = 508 m/s Q1.9 Using the results of the previous questions, determine the mean time between collisions of air molecules. l 2.47x108 m 4.86x1011 s v 508m / s Q1.10 Estimate the diffusion constant of the air molecules 1 1 D l v 2.47 x10 8 x508 1.71x10 6 m 2 / s 3 3 Q1.11 Discuss how the results above would change if the temperature was varied while keeping the pressure and volume in the room constant. The velocity of air molecules will increase. Hence, there will be a decrease in the mean free time and increase in diffusion constant Q2. Name two key advantages of olfaction with regards to vision? Permanence Odors is more permanent than light in the sense that a scent will remain for some time even after the scent source has moved away while object can no longer be seen when it has been moved away Manufacturability Most organisms produce odor but not light because many odors are simple organic compounds. Detection The spatial resolution of detected light is more complicated hence the development of adequate visual system is more complex than that of olfactory system. Odor can be useful in places where light cannot pass such as muddy water, soil etc Odor detection works as well in the dark as it does in the light Q3. We have discussed odors that spread through air. Could they spread through water? What does this mean for fish? Yes, odors spread through water. The diffusion of odors in water and in fact in any medium occurs through brownian motion (random collision between the odorant and the medium particles. Since diffusion is possible in water, olfaction sensor is quite highly developed for some fishes for example shark which can smell as little as one part per million of blood in water Q4. On an inhabitable planet orbiting a significantly hotter star than our sun, would the leaves more likely be blue or red? A hotter star will have a spectrum with an intensity peaks shifted toward shorter wavelengths. Hence, we should expect that the leaves would more likely be blue. Whether or not a leaf would really be blue depends on many factors which likely cannot be answered until we discover other planets with life. Q5. How many photons does it take to split one water molecule? Since two electrons need to be removed from two hydrogen atoms in water then only two photons are necessary namely one for each electron harvested . According to this website http://www.biologie.uni-hamburg.de/bonline/e24/24c.htm , there is only one photon needed to split two water molecules Q6. What would the color be of fluorescent chlorophyll molecules? In fluorescence, light is absorbed and then later emitted. In most cases, the fluorescent light is only emitted when the molecule returns from the first (lowest) excited state to the ground state. If it was excited to a higher level than first excited state, usually, the molecule first loses some energy due e.g. rotation or vibration bringing it to the first excited state. Fluorescent chlorophyll In the case of chlorophyll, there are two clear peaks in the absorption. The one corresponding to the longer wavelength, i.e. the peak around 650 nm (red light) is associated with the lowest excited state and therefore gives the color of fluorescent chlrocphyll In short: fluorescent chlorophyll is red jellyfish Q7. If an atom would have three different excited levels, how many different wavelengths can the absorbed and emitted photons have? T23 There are 6 possible wavelengths as shown in the figure T12 Ground state Q8. Why are there two different lines in Figure 12.10? Chlorophyll has a number of (closely related) different structures. Each structure has a slightly different absorption spectrum. Most terrestial plants have chlorophyll a Q9. Is it conceivable to have a photosynthesis-like process in leaves that are transparent of visible light (that is, the visible light cannot be used as an energy source)? Yes, it is feasible. Whether it would be biologically useful is another matter though. It may be reasonable to speculate that the wavelength should not be too far from visible range because a too long wavelength may not have enough energy to do something practically useful and on the other hand the energy of a too short wavelength may be so large that it destroys the photosynthesizing molecules. Moreover, the quantity of photons is also important to light. Hence, there is an evolutionary benefit in having photosynthesis using visible light since the maximum intensity of sunlight is in the visible range.