Dissolution and Solubility Processes
advertisement
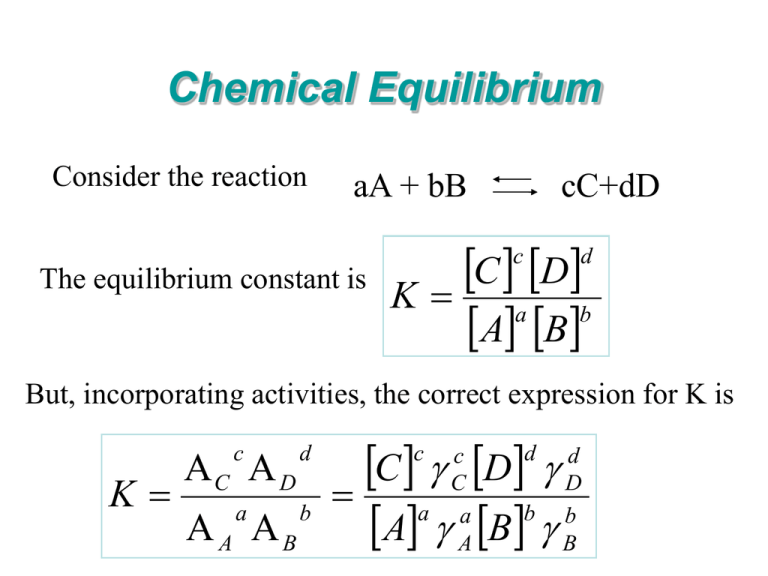
Chemical Equilibrium Consider the reaction aA + bB cC+dD C D K a b A B c The equilibrium constant is d But, incorporating activities, the correct expression for K is K c C D d A B b a C D b A B c c C a a A d d D b B Thermodynamic dissolution constants CaSO4.2H2O Ca+2 + SO4-2 + 2H2O • • • • Account for activities, not just concentrations If solid is ‘pure’, its activity = 1; The activity of water = 1 E.g., Kdis for gypsum: 1 Kdis = (Ca+2)(SO4-2)(H2O)2 (gypsum) 1 Kdis = (Ca+2)(SO4-2) = Kso Kso = 2.4 x 10-5 for gypsum (~10-4.62) Ion Activity Product (IAP) ML(s) M+aq + L-aq Gypsum Ca+2 + SO4-2 + 2H2O Kso = 10x Kso = 10-4.62 IAP (M+)(L-) = Kso at equilibrium. Kso is the maximum IAP a solution will tolerate without precipitation. If IAP > Kso then precipitation occurs and if IAP < Kso then dissolution occurs Use IAP and Kso to determine the relative saturation of a solution: • IAP/Kso = 1 then soil solution is in equilibrium with the solid • IAP/Kso < 1 then soil solution is undersaturated with respect to the solid phase and the solid will continue to dissolve • IAP/Kso > 1 then soil solution is saturated with respect to the solid phase and secondary minerals may precipitate (depending on kinetics) IAP/Kso is also related to the saturation index, SI: • SI = log IAP/Kso • When SI = 0, then soil solution is at equilibrium (IAP = Kso and IAP/Kso = 1 and log 1 = 0) • When SI < 0, then solution is undersaturated (solid dissolves) • When SI > 0, then solution is supersaturated (solid precipitates) Common Ion Effect • A salt is generally less soluble in a solution containing an ion which is the same as one of the constituent ions of that salt. • Le Chatelier's Principle: "If a system in equilibrium is subjected to a stress the equilibrium will shift in the direction which tends to relieve that stress." (and return to equilibrium) • (M+)(L-) = Kso Gypsum dissolution in water Gypsum + water Ca2+ + SO42- + water More Ca+2 or SO42- in solution drives eqn to the left = lower solubility Solubility of gypsum Common Ion effect on Gypsum solubility MgSO4 CaCl2 Concentration Ion-pairing • aka ion activity or ionic strength effect • Mineral solubility is enhanced or increased when different ions are added to the solution forming ion pairs and complexes • Gypsum + MgCl2 Ca2+ + SO42- + Mg2+ + 2Cl- + CaCl+ + CaCl20 + MgCl+ + MgSO40... • As ionic strength increases, solubility increases • Dissolution reaction is driven to the right (solubility increases) Ion-pairing or Ion activity effect on gypsum solubility Solubility of gypsum MgCl2 NaCl Concentration