Wulff Construction
advertisement
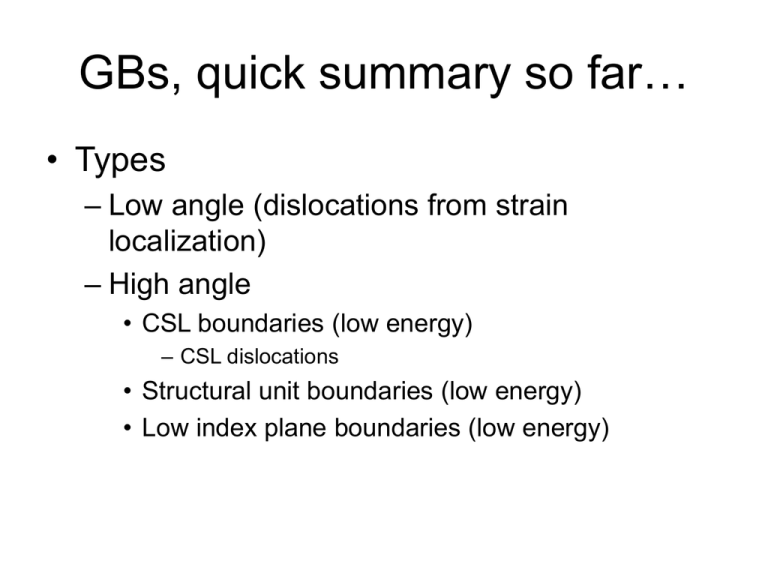
GBs, quick summary so far…
• Types
– Low angle (dislocations from strain
localization)
– High angle
• CSL boundaries (low energy)
– CSL dislocations
• Structural unit boundaries (low energy)
• Low index plane boundaries (low energy)
But
• This only addresses energy versus
tilt/twist, what about plane?
Wulff Construction
• The standard approach is to consider how a
property scales as a function of the size of the
system (R). In a generic sense one can write:
• P(R) = AR3 + BR2 + CR + D
• A => Bulk behavior
• B => Surface/Interface term, or at least what
scales as a surface term
• C => Edge/Line term
• D => Limit for atomic behavior
• The bulk properties of a material only depend
upon “A”; but we have additional terms.
Example
• If P(R) is a free energy
• B = surface free energy (for a surface); interfacial free
energy, grain boundary free energy or stacking fault free
energy. Normally use g
• C = dislocation free energy (line)
• D = point defect free energy (zero dimension)
• P(R) is an entropy – similar
• Other things as well. For instance dE/de (e a strain) is
the stress in the bulk. Similarly we can discuss can write
dg/de as an interfacial/surface stress term, or a line
stress term for a dislocation.
Method
• In the west, proof is generally attributed to
Conyers Herring, but a more correct
attribution is to Von Laue during the 2nd
world war
Max Von Laue
Conyers Herring
Approach
• Write the problem as minimizing the total
surface free energy as a function of what
surface facets are present, for constant
volume:
• Minimize
– F = S giMi - l(1/3)S miMi
– Note: Lagrangian
• Solution
– gi = lmi
From g-plot to EQUILIBRIUM SHAPE OF CRYSTAL → the Wulff construction
Draw radius vectors from the origin to intersect the Wulff plot (OA in Figure)
Draw lines to OA at A (line XY)
The figure formed by the inner envelope of all the perpendiculars is the
equilibrium shape
Example
Gold Octahedra
• Polyol synthesis developed by Oh Cho group
• Synthesized by Mirkin group
• {111} capped, single crystal
VertexAndEdgeTruncationsOfThePlatonicSolids.nbp
C. Li, et al., ACS Nano. 2, 1760 (2008)
Gold and Silver Cubes
Au
VertexAndEdgeTruncationsOfThePlatonicSolids.nbp
Crystal shape of pure Cu and of Bi-saturated Cu at ~ 900°C (with
monolayer of adsorbed Bi at the surface) illustrates effects of
segregation on ECS
Cu
Bi-saturated Cu
Curtesy Paul Wynblatt
Example: scanning electron microscope image of a Bi-saturated Cu
"negative" crystal
Curtesy Paul Wynblatt
Morphology of Pb
crystals as a function
of T
Facets
Curtesy Andrew Zangwill
SrTiO3 cubes
VertexAndEdgeTruncationsOfThePlatonicSolids.nbp
• {110} facet stabilization: cubo-octahedral shape.
Wulff & Winterbottom
001
001
100
γ111√(3/2)
110
γ111
γ111
γ100
γInt – γSub = γPt
Increasing γint
0 < γInt – γSub < γPt
γInt – γSub = 0
-γPt < γInt – γSub < 0
γInt – γSub ≤ -γPt
Increasing γsub
16
Increasing γPt
Modified Wulff Construction
(twins)
Kinetic Wulff construction
If, instead of the surface/interface free
energy we use growth velocity, a quasistationary kinetic shape is generated by
exactly the same construction
Often the case when kinetics dominate