Bond angles
advertisement

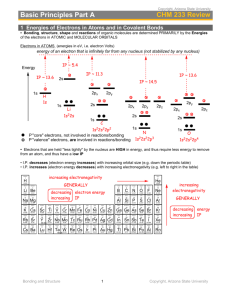
Bond angles The standard angles are as follows: ► Linear: 180 ° ► Triangular planar: 120 ° ► Tetrahedral: 109.5 ° ► Trigonal bipyramidal: 90 ° (between vertical and horizontal planes) 120 ° (within the horizontal plane) ► Octahedral: all 90 ° Why? ► The major thing that effect bond angles is The number of areas of electron density around the central atom – 2=180 °, 3= 120 °, 4= 109.5 °, 5= 120 and 90 ° and 6=90 ° Minor changes due to nonbonding electrons ► When there are non-bonding electrons, they are held closer to the central atom as there is no other atom pulling on the electrons. Therefore repulsion is increased, and the bond angle between the bonded atoms decreases. ► For example, water, methane and ammonia all have 4 areas of electron density and so are basically tetrahedral. However, methane has no non-bonding electrons, ammonia has one pair and water has two. ► The bond angles are: methane – 109.5 °, ammonia - 107 °, and water – 105 °. Question ► Rank the following compounds in terms of the bond angle (highest to lowest) NO2, NO2-, NO2+ Answer ► Highest to lowest: NO2+ > NO2 > NO2- ► Reasons: NO2 has a single non-bonding electron which repels less than a pair of non-bonding electrons in NO2- . Both have 3 areas of electron density and so the bond angle is approximately 12o degrees. NO2+ has only 2 areas of electron density and so its bond angle is 180 degrees. Question ► Draw the structural formula for methyl ethanoate. Label the following bond angles: H-C-H, O-C=O, and C-O-C. Answer ► H-C-H - tetrahedral 109.5 ° ► O-C=O - triangular planar 120 ° ► C-O-C - tetrahedral with non-bonding pairs approx 105 °. Bond lengths ► 1. 2. Rank the following bonds in order of length, longest to shortest: C-C, C=C and C=C C-C, H-H and Cl-Cl Answers C-C > C=C > C=C Reason: More electrons in a multiple bond means a stronger attraction for both nuclei and therefore a shorter bond. 2. Cl-Cl > C-C > H-H Reason: Bond length is the distance between the centre of the 2 nuclei. As Cl has more electron shells, the distance between the 2 Cl nuclei is greater than that of C and H. 1.