Calculating Protein Charge
advertisement
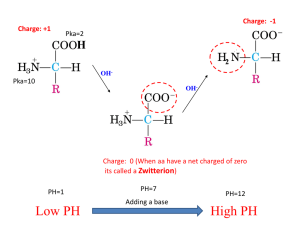
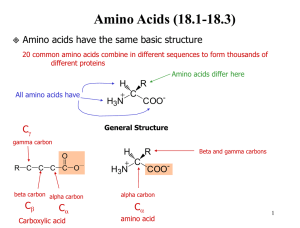
Calculating Net Protein Charge The Problem • A protein’s net charge depends on the number of charged amino acids it contains and the pH of its environment. – The isoelectric point is the pH at which the net charge is zero. • Each amino acid has a different dissociation constant, KD. – The amino acids that can be ionized at physiological pHs are: aspartate (D), glutamate (E), lysine (K), arginine (R), and histidine (H). – Also, the N and C termini can be ionized. • • The important point is: most of these amino acids are not fully ionized or fully unionized at physiological pHs. It is your job to calculate the proportion of each amino acid that is ionized at a given pH. Once this is done, calculating the net charge is straightforward. – Multiply the proportion by the number of that amino acid, taking into account the differences between acidic (-COOH) and basic (-NH2) types. Amino Acids Contributing to Charge • Charged amino acids come in two forms: • Acidic amino acids (and the C-terminal) are in the –COOH form (uncharged) at low pH and in the –COO- form (-1 charge) at high pHs. – These include aspartic acid (D) and glutamic acid (E) • Basic amino acids (and the N-terminal) are in the –NH3+ form (+1 charge) at low pH and in the –NH2 form (uncharged) at high pH. – These include lysine (K), arginine (R) and histidine (H). • All other amino acids do not affect charge under physiological conditions. Dissociation Constant • The association and dissociation of the H+ with the amino acid is governed by its dissociation constant, KD. • Dissociation constant is the ratio of the concentration of the dissociated forms to the concentration of the combined form. – Each amino acid has a separate dissociation constant. – The equation below uses [R-] to indicate the concentration of the amino acid when the H+ is dissociated from it. • The pK value is analogous to pH. – pK = -log10KD – KD = 10-pK [ H ][R ] KD [ RH ] Calculating the Proportion with H Associated • Starting with the dissociation constant equation, we need to derive an equation for the proportion of amino acid molecules that have H associated at a given pH. We rearrange to get: [ H ][R ] [ RH ] KD Substituting this into the proportion equation: [ H ][R ] KD proportion [ H ][R ] [R ] KD Some algebraic manipulation: We want: [ RH ] proportion [ RH ] [ R ] Start with the definition of dissociation constant: [ H ][R ] KD [ RH ] [ H ][R ] proportion [ H ][R ] K D [ R ] Our final result: [H ] proportion [H ] K D Acidic and Basic Amino Acids • The proportion equation calculates the proportion of a given amino acid that has H associated with it at a given pH. – Recalling that [H+] = 10-pH and pH = -log10[H+] • For the basic amino acids (H, K, and R) and the N-terminus, the form with H associated is –NH3+, which has a +1 charge. – Thus, the contribution to net charge for each of these amino acids is just the number of each amino acid multiplied by the proportion that has H associated. • For the acidic amino acids (D and E) and the C-terminus, the form with H associated is –COOH, which is uncharged. Thus, the proportion that is in the charged form ( -COO-) is 1 minus the proportion with H associated. – Also, remember that the charged form has a negative charge, The net contribution from acidic amino acids needs to be subtracted from the contribution of the basic amino acids. Summary 1. Calculate the proportion of each ionizable amino acid (plus the N- and C-termini) that have H associated at the given pH. 2. For the acidic amino acids, calculate the percentage that are charged by taking one minus the proportion with H associated. 3. Multiply the proportion charged by the number of each amino acid present in the protein. 4. Subtract the negative charge total from the positive charge total to get the net charge.