lecture_2-1
advertisement

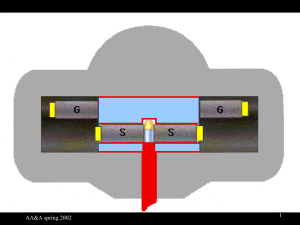
AA&A-Spring 2002 1 t years after death • • • • NO CO2 exchange with atmosphere C12 is stable C14 decays Ratio at time t is reduced to Rt with Rt = C14/C12 at time t = R0 (1/2)t/T (T = T1/2) = R0 e-t/T (T = Tm) • t = T1/2 log2(R0/Rt)= Tm ln(R0/Rt) with T1/2 = 5568 years or Tm = 8033 years AA&A-Spring 2002 2 How to measure Rt = C14/C12 ratio • Use chemistry to convert sample to known compound of carbon of high purity • Weight gives quantity of C12 (and a little C13) – NC12 = 6 x 1023 x (weight of carbon in grams)/12 • Radioactivity gives quantity of C14 – Carbon compound dissolved in liquid scintillator – Decay electron stimulates light emission by scintillator – Light pulses converted to electrical pulses by photomultipliers and counted at rate D per minute • NC14 = D x Tm(in minutes) = D x (Tm x 365 x 24 x 60) AA&A-Spring 2002 3 Counting C14 activity C14 Electron path Photons (light) photomultiplier photomultiplier Sample cell AA&A-Spring 2002 4 ****Critical for naïve approach**** • Work from: t = T1/2 log2(R0/Rt)= Tm ln(R0/Rt) • R0 = C14/C12 at death is known – C14 production independent of time – C12 content of reservoir independent of time – Mixing adequate to give SAME C14/C12 everywhere • C14/C12 in organism same as in environment • Rt = C14/C12 at time of dating is known • T1/2 is known • LET’S BRAINSTORM! HERE COME QUESTIONS: NOT STATEMENTS OF WHAT’S IMPORTANT!! AA&A-Spring 2002 5 *C14/C12 ratio independent of time?* • Both changes in earth’s magnetic field and changes in magnetic field at the earth due to solar activity change the magnitude of the cosmic rate intensity, hence generation rate of C14 • Nuclear bomb testing in ‘50s doubled C14 concentration in atmosphere • How much release of C14 from nuclear reactors? • Fossil fuel burning releases ONLY C12 into the atmosphere: lowered C14/C12 ratio by ~1% by 1950 • Volcanic release of carbon species might do the same • Effect of global climate change (e.g., ice age)? AA&A-Spring 2002 7 *C14/C12 ratio everywhere the same?* • Is mixing between the stratosphere (where C14 is produced) and the troposhere really fast enough? • Is mixing equally efficient at all latitudes • If nutritional cycling of carbon is important mixing mechanism, polar regions may be poorly mixed • Mixing of deep with shallow ocean water is NOT all that fast. (Deep water may be “1500 years old” in C14 time.) • Surface waters (ponds and streams) containing CaCO3 leached from limestone (in which any original C14 will have long since decayed) will have anomalously low C14/C12 ratio AA&A-Spring 2002 9 *****C14/C12 ratio in organism same as local reservoir?***** • Chemical fractionation? Chemical (and biological) reaction rates ARE a little different for different isotopes. Not important UNLESS isotope ratio is critical issue • Do all parts of the organism have the same C14/C12 ratio? (E.g., trees) • Which part of the reservoir, the air it breathes or the water it drinks? • How long does it take a tree to die? AA&A-Spring 2002 12 **t = T1/2 log2(R0/Rt)= Tm ln(R0/Rt)** • Do we know R0? See last few slides. • Have we measured Rt correctly? More next Wednesday. • Is the decay really exponential? Yes, to the best we can measure. • Does T 1/2 depend on chemical environment? Not in any significant way. • Is T 1/2 = 5568 years? (= Libby half-life) NO! Better is T 1/2 = 5730 years (= Cambridge half-life) AA&A-Spring 2002 14 Calibration graph Calibration graph to convert from conventional radiocarbon age to calibrated calendar date. AA&A-Spring 2002 17 Implications • NOT that method is invalid • Ideas point to where care is required – Care in technique – Care in interpretation • Need for knowledge of other disciplines • YOU HAVE TO KNOW WHAT’S BEHIND THE KNOBS AA&A-Spring 2002 18