I-2
advertisement
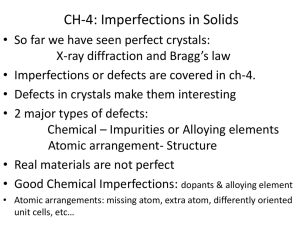
Defects in crystals, Non-stoichiometry Point defects Vacancy X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X Point defects Interstitial X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X Point defects Substitutional impurity X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X Y X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X Point defects Schottky pair + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + + - + - + - + - + - + - + - + - + - + - + - + - - + - + - + - + - + - + - + - + - + - + - + - + Point defects Frenkel defect + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - + - - + - + - + + - + - + - + - + - + - + - + - + - + Other defects Line defects • Edge dislocation • Screw dislocation Plane defects • Stacking fault • Small angle grain boundaries Non-stoichiometry Example: FeO Stoichiometric case: Fe2+ : O2- = 1:1 Usually: partial oxidation of Fe2+ occurs non-stoichiometric composition: Fe1-xO Say for example: Let: [Fe3+] = a ; Fe3+/Fe2+ = 0.05 [Fe2+] = b a/b = 0.05 charge balance : 3a + 2b = 2 Solving : a = 0.05; b = 0.93 Therefore the non-stoichiometric composition = Fe0.98O Effect of defects on properties Frenkel defects • AgBr, AgCl • ionic conduction, photography Substitutional impurities • Extrinsic semiconductors • n-type: P doped Si • p-type: B doped Si Relevance of non-stoichiometry • • • • electrical conductivity corrosion electrode surface activity catalysis Examples of non-stoichiometric materials • Tungsten bronzes: NaxWO3 colored materials • 123 oxides: YBa2Cu3O7-x high Tc superconductors Problem Set 1. The average energy required to create a vacancy in a monatomic solid is 1.1515 eV/mol. Compute the ratio of vacancies at 1000K and 500 K. 2. How are the density of crystals affected by the presence of Schottky and Frenkel defects ? 3. Addition of Ca2+ increases the conductivity of KCl crystal. However, it is found that the conductivity is still due to the motion of K+ ions rather than the doped Ca2+ ions. Discuss the mechanism for the enhanced conductivity. 4. If the ratio of A3+ to A2+ in a nonstoichiometric sample of an ionic crystal AO is 0.15, what is the nature of the defect ? Derive the correct formula for AO. 5. Discuss the nature of nonstoichiometry in (a) copper sulfide, (b) zinc oxide, (c) praseodymium oxide. What are the different structures in the case of (c). 6. NiTe exhibits nonstoichiometry. Draw schematic diagrams to illustrate the crystal structures of the two extreme formulations.