Lecture Thermodynamics
advertisement

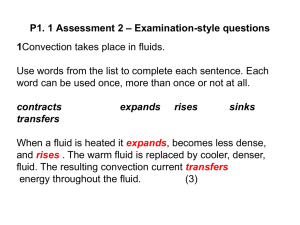
TEMPERATURE AND HEAT As close to chemistry as we can get Temperature Temperature and heat are often used interchangeably Temperature is a measure of how “hot” or “cold” an object is Thermometers are objects that measure temperature. Thermometers work by putting them in contact with the object whose temperature we want to measure and waiting until reading is steady Thermometer has reached Thermal Equilibrium Zeroth Law of Thermodynamics If A is in thermal equilibrium with C and B is in thermal equilibrium with C, then A and B are in thermal equilibrium Two objects are in thermal equilibrium if and only if they have the same temperature. A thermometer actually measures its own temperature. Temperature Scales Celsius Water Freezes at 0, Boils at 100 Fahrenheit Tf Kelvin 9 5 T C 32 Gas thermometers use the pressure of the gas to determine temperature. At -273.15oC the pressure of the gas would reach zero DON’T EVER USE “degrees” Kelvin Linear Expansion Materials expand when heated (exceptions include water) If the difference in temperature is not too big, the change in length is directly proportional to the change in temperature L L0 T Where α is the coefficient of thermal expansion (K-1) Linear and Bulk Expansion β is the coefficient of volume expansion (K-1) For solids β=3α Serway 19-18 At 20.0oC an aluminum ring has an inner diameter of 5.000 cm and a brass rod has a diameter of 5.050 cm. (a) If the ring is heated, what temperature must it reach so that it will just slip over the rod? (b) What if? If both were heated together, what temperature must they both reach so that the ring slips over the road? Would the latter process work? Holes expand with the material L L0 T L L 0 (1 T ) a ) 5 . 050 5 . 000 [1 24 . 0 x10 6 C 1 (T 20 . 0 C )] T 437 C b ) 5 . 050 [1 24 . 0 x10 6 C 1 (T 20 . 0 C )] 5 . 000 [1 19 . 0 x10 T 3000 C Aluminum melts at 660oC 6 C 1 (T 20 . 0 C )] Heat When you put a warm object in thermal contact with a cold object, energy will transfer from the hot object to the cold one. This energy transfer is called heat flow, or heat. unit of heat calorie (cal) Symbol is Q Defined as the amount of heat required to raise the temperature of 1 gram of water from 14.5oC to 15.5oC. 1cal =4.186J (SI unit still J) A food value calorie is actually 1 kcal Specific Heat Specific Heat, c, of a material to is the quantity of heat needed to raise the temperature of a material of mass m from T1 to T2. It is proportional to ∆T. Molar Heat capacity Sometimes its easier to use number of moles rather than mass. n is the number of moles M is the mass per mole Q mc T c m nM 1 dQ Q nMc T m dT Q nC T Specific heat capacity of water is 4190 J/kgK C 1 dQ n dT Mc Phase changes Phase – specific state of matter (solid, liquid, gas) Phase change or phase transition – when matter changes from one phase to another Phase change occurs at a specific temperature and pressure Ex. Water freezes at 0oC and 1 atm Latent Heat of Fusion Given 1kg of Ice at 1 atm, if we begin heating it slowly, the temperature of the ice will not increase above 0oC until all the ice has melted – it has achieved phase equilibrium Latent Heat of Fusion – Lf, is the amount of heat needed per unit mass to convert matter from solid to liquid (ex. Lf of ice is 3.34x105 J/kg) Q melt mL f same amount of heat is needed to freeze object Q freeze mL f Latent Heat of Vaporization Same as before except for liquid to gas (Lv of water is 2.256x106 J/kg) Q mL v Supercooling Supercooling – very pure liquid can be cooled below its freezing point, resulting in an unstable state. Any slight agitation or crystal can cause the liquid to turn to solidify Superheating Superheating – very pure liquid can be heated above its boiling point, resulting in an unstable state. Any slight agitation will cause bubbles to form. It usually causes boiling liquid to be flung everywhere so be careful when heating water in a microwave Example What mass of steam, initially 130oC, is needed to warm 200g of water in a 100g glass container from 20.0oC to 50.0oC Example Heat lost by steam will be heat gained by the water Q cold Q hot Steam will cool, become water, and cool once more 130-100 Q mc T Q m s ( 2010 )( 100 130 ) Q m s ( 6 . 03 x10 ) 4 Steam becomes water Q m s ( 2 . 26 x10 ) 6 Water 100-50 Q m s ( 4190 )( 50 100 ) Q m s ( 2 . 09 x10 ) 5 Example Total Q Q Total m s ( 2 . 53 x10 ) 6 Q cold Q hot Q cold Q water Q glass Q cold 0 . 2 ( 4190 )( 50 20 ) 0 . 1( 837 )( 50 20 ) Q cold 2 . 77 x10 J 4 2 . 77 x10 J m s ( 2 . 53 x10 ) 4 m s 10 . 9 g 6 Mechanisms for Heat Transfer Conduction – occurs between objects in contact. Hotter atoms will have more kinetic energy and will jostle neighbouring atoms, giving some of their energy. Atoms don’t move. Metals are good conductors because free electrons transfer energy as well Conduction Heat will flow from higher to lower temperatures. Heat current, H - amount of heat transferred per unit time (J/s) H dQ dt kA T H TC L A is cross sectional area of contact, L is the lenght. T T C is the H L temperature gradient Conduction Constant k depends on the material. Higher k, better conductor Thermal resistance, Ropposite to conduction H dQ A T H TC dt R R L k Example Two slabs of thickness L1 and L2 and thermal conductivity k1 and k2 are in contact with each other as shown. Determine the temperature at the interface and the rate of energy transfer by conduction. Assume steady state conduction Example Steady State conduction just means that energy flows at one rate all through out. H 1 k1 A H 2 k2 A T TC L1 TH T k1 A T TC L2 T H L1 k2 A TH T L2 K 1 L 2 T c K 2 L1T H K 1 L 2 K 2 L1 A (TH TC ) L1 k1 L2 k2 Convection Transfer of heat by mass motion of a fluid (ex. Air) Hot air rises and cool air sinks. The process is complicated (due to turbulence) and no easy equations exist. Heat current due to convection proportional to surface area Viscosity of a fluid slows down convection Heat current is proportional to 5/4 power the temperature difference between the surface and the main body of the fluid. Radiation Transfer of heat through electromagnetic waves Best example, heat from the sun. Everything emits energy in the form of EM radiation H Ae T 4 Where e – emissivity, ratio of rate of radiation from a surface to an ideal surface σ-Stefan-Boltzmann Constant = 5.670400(40)x10-8 W/m2K4 Radiation and Absorption While an object is radiating, it will also absorb heat. H net Ae (T T s ) 4 4 If e is 1, that means it is an ideal absorber/radiator of energy. Called a black body If e=0, it is an ideal reflector Serway 20.47 The sun has a surface temperature of about 5800K. The radius of the sun is 6.96x108m. Find energy radiated by the sun per second. e=0.965 Young and Freedman 17.25 A glass flask with a volume of 1000.00cm3 a at 0.0oC is completely filled with mercury. When the flask and the mercury are heated to 55.0oC, 8.95cm3 of mercury overflows. If coefficient of volumetric expansion of mercury is 18.0x10-5K-1, What is the volume expansion of the flask? Giancoli 14-18 A 1.20 kg hammer head is travelling at 6.5 m/s before it strikes a 14.0 g nail and is brought to a rest. Assuming the nail absorbs all the energy, what is its temperature after 10 such hammer blows. Young and Freedman 17.60 A glass vial containing a 16.0 g sample of an enzyme cooled in an ice bath. The bath contains water and 0.120kg of ice. The sample has specific heat of 2250J/kgK; the vial has mass 6.00g and specific heat 2800J/kgK. How much ice melts in cooling the enzyme from room temperature (19.5oC) to the temperature of the ice bath. Young and Freedman 17.70 A long rod, insulated from the sides is in perfect contact with boiling water and an ice water mixture. The rod consists of 1.00m section of copper and a length L2 of steel. Both sections have a cross sectional area of 4.00cm2. The temperature of the copper/steel junction is 65.0oC after steady-state has been setup. (a) How much heat per second flows from boiling water to the ice-water mixture?(b) What is the length of L2?