Discussion 1 - Loy Research Group
advertisement

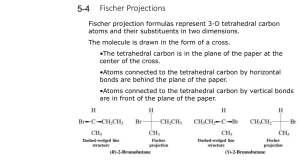
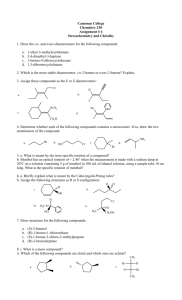
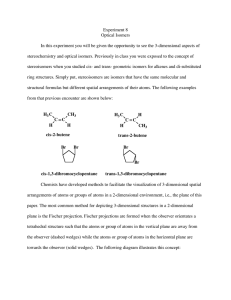
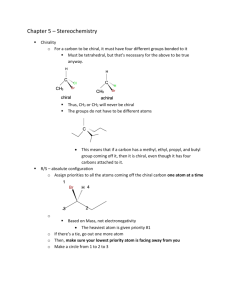
Discussion Carbohydrates Nomenclature (D)-glucose (2R,3S,4R,5R)-2,3,4,5,6-pentahydroxyhexanal α-pyranose form: α-(D)-glucopyranose Dextrose Hawthorne structure α-pyranose form: α-(D)-glucose β-pyranose form: β-(D)-glucopyranose Another Chiral sugar: Erythrose How do you convert to a regular zig-zag structure? Three ways: 1) use models 2) determine R or S configurations, draw a four carbon chain and draw substituents then recheck configurations 3) Draw intermediate structures and rotate molecule and bonds to get into zig-zag Method 2: Method 3: By drawing & rotating Takes time, can make mistakes and you still should check the configurations (R or S) at the end Draw the enantiomer of erythrose Draw the enantiomer of erythrose You are switching two groups –involves breaking and making two bonds – to interconvert each center (Four bonds total) Draw the diastereomers of the erythrose isomers shown To create diastereomers 1) first figure out how many total possible diastereomers ( = 2n, n = # chiral carbons). There are 22 = 4 diastereomers possible (two pair of enantiomers) 2) then switch two groups to just one of the chiral centers to form one diastereomer. 3) then draw its mirror image as above. Meso compounds Found among diastereomers of molecules with plane of symmetry between two (or four, six, eight… ) chiral centers. With this plane of symmetry the RS & SR enantiomers are not enantiomers but are superimposable. These are achiral and will not show optical activity. Meso is an issue with stereoselective reactions (syn or trans) to symmetrical olefins To generate 1,2-dihalides, 1,2-diols, epoxides or cyclopropanes For example: [1] If the ring closes on a hydroxyl which is on the right in the Fischer projection, the hydroxymethyl group (tail) points up; if it closes on a hydroxyl which was on the left in the Fischer projection, the tail points down. [2] The ring hydroxyls point down if they are on the right in the Fischer projection, and up if they are on the left in the Fischer projection. (Note that these are also the positions they are in after the Fischer projection has been rotated 90° clockwise to lie on its side.) [3] The hydroxyl on the anomeric carbon points down in the D series if it is α and up if β In the L series, α is up and β is down Nomenclature ? Nomenclature (D)-fructose α-(D)-fructofuranose (2S,3S,4S,5R)-2,5bis(hydroxymethyl)tetrahydrofuran-2,3,4-triol (2S,3S,4S,5R)-2,5bis(hydroxymethyl)tetrahydrofuran-2,3,4-triol S R R S S S R Nomenclature (D)-fructose Sucrose Fisher Projections Epimers Optically active Optically active C2 epimers C2 epimers C2 epimers HNO3 Optically active Mirror image is same compound One segment of Fischer Proof