5-Intro 2 Catalyst - Dicky Dermawan
advertisement

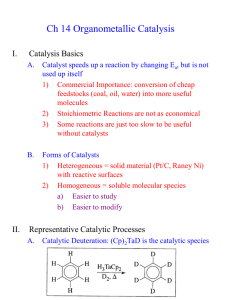
ITK-329 Kinetika & Katalisis Chapter 5 Introduction to Catalyst & Catalysis Dicky Dermawan www.dickydermawan.net78.net dickydermawan@gmail.com 88 Most chemical processes use catalyst at some stage in production process Catalyst (Ostwald): A substance one adds to a chemical reaction to tremendously speed up the reaction without the catalyst undergoing a chemical change itself Actually catalysts do undergo chemical changes during the course of reaction. It’s just that the changes are reversible. Catalyst: • Selectively enhances the rate of a reaction • Changes the reaction pathway • Neither consumed nor formed • Does not affect equilibrium 89 Types of Catalysts Homogeneous Catalyst • Acids or bases • Metal salt • Enzymes • Radical initiators • Solvents Reaction C2H4 Polyethylene C2H4 Polyethylene, C6H5CH=CH2 Polystyrene C2H4 + ½ O2 CH3CHO (Wacker process) Olefins + CO +H2 aldehydes (hydroformylation) CH3OH + CO CH3COOH SO2 + ½ O2 SO3 (lead chamber process) CH3COOH + CH3OH CH3COOCH3 + H2O Sucrose glucose + fructose Catalyst TiCl4/Al(C2H5)3 (Ziegler – Natta catalyst) Peroxides PdEt3 Co(CO)6 RhCl3 NO/NO2 Acids or bases Invertase 90 Types of Catalysts (cont’) Heterogeneous Catalyst Catalyst Pt on alumina,Ni on alumina Pt/Sn on acidic alumina Solid acids (zeolites) Ag V2O5 Platinum gauze Reaction Hydrogenation/dehydrogenation Reforming Hydrocarbon isomerization, cracking C2H4 + ½ O2 ethylene oxide SO2 + ½ O2 SO3 2 NH3 + 4 O2 N2O5 + 3 H2O High surface area 91 How Catalysts Work 92 How Catalysts Work (cont’) Catalyst can initiate reactions. The mechanisms are similar to the mechanism without a catalyst, but the initiation process is much faster with the catalyst NO CAT ALIST WIT HCAT ALIST Init iat ion: Init iation: k 1 C 2 H 6 2CH 3 1 NO 2 C 2 H 6 C 2 H 5 HNO2 P r opagat ion: P r opagat ion: k k 2 CH 3 C 2 H 6 CH 4 C 2 H 5 k3 C 2 H5 C 2 H 4 H k4 H C 2 H 6 C 2 H5 H 2 T ermin at ion: k5 2C 2 H5 C 4 H10 k 3 C 2 H 5 C2H 4 H k 4 H C 2 H 6 C 2 H 5 H 2 T ermin ation: k 5 2C 2 H 5 C 4 H10 Radical Initiator in polymerization: peroxides ROOR, AIBN [(CH3)2C(CN)N]2 93 How Catalysts Work (cont’): Intermediate Stabilization Reactant + Catalyst Stable Complex Products + Catalyst Acid catalysts: CH3CH2HC=CH2 + H CH3CH2HC=CH2 + H+ CH2 + H CH3CH2HC CH3HC=CHCH3 + H+ CH2 CH3CH2HC CH2 + H CH3CH2HC CH3CH2HC CH3HC=CHCH3 + H CH3CH2HC=CH2 + H+ H CH2 CH2 =CHCH2CH3 + H+ 94 How Catalysts Work (cont’): Intermediate Stabilization Reactant + Catalyst Stable Complex Products + Catalyst Enzymatic Reactions: O NH2CNH2 + HOH 2NH3 + CO2 95 How Catalysts Work (cont’): Intermediate Stabilization Reactant + Catalyst Stable Complex Products + Catalyst Metalic cluster catalyst: HI,[ Rh ( CO ) 2 I 2 ] CH 3 OH CO CH 3COOH 96 How Catalysts Work (cont’): Intermediate Stabilization Reactant + Catalyst Stable Complex Products + Catalyst Gas phase, no catalyst: On solid Pt(111) catalysts: 108 enhancement factor 97 How Catalysts Work (cont’) Example in Heterogeneous Catalysis Ethylene hydrogenation to ethane on Ni catalyst Heterogeneous Catalysis = adsorption – (surface) reaction - desorption 98 Heterogeneous Catalyst Activity: Turnover Number/Turnover Frequency Tn = the rate that molecules are converted per active site in the surface of the catalyst per second. Tn [] molecules/ sec R A [] sec 1 surface_ atom N s 99 How Catalysts Work (cont’): Configuration Dependent Pd 3C 2 H 2 C 6 H 6 Zeolite CH 3 C 6 H 5 CH 3 OH CH 3 C 6 H 4 CH 3 H 2 O Molecule Platinum surface structure Linear alkane Isoalkane Benzene Paraxylene Ortoxylene 2-Methyl alkenes Naphtalene Zeolite Chabazite Zeolite A Erondite Ferrierite ZSM-5 Offretite Mordenite Faugasite VFI Size of Diffusion Channel, ? 3.6 x 3.7 4.1 x 4.1 3.6 x 5.2 4.3 x 5.5 5.5 x 5.6 6.4 x 6.4 6.7 x 7.0 7.4 x 7.4 13 x 13 Min. Diameter, ? 4 5.5 5.1 5.1 5.7 5.1 7.3 Size of Cavity, ? 5 6.5 11.6 6.5 10.5 6.5 10.5 11.9 100 How Catalysts Work (cont’) Catalyst lowers the activation barrier for the reaction, Reaction Catalyst Ea, Ea’, kcal/mol kcal/mol 44 14 500K Rate enhancement 1013 H2 + I2 2 HI Pt 2 N2O 2 N2 + O2 Au 58 29 1013 (C2H5)2O 2 C2H4 + H2O I2 53 34 108 Reaction Catalyst Rate Temperature enhancement 1040 300 ortho H2 para H2 Pt (solid) 2 NH3 N2 + 3 H2 Mo (solid) 1020 600 C2H4 + H2 C2H6 Pt (solid) 1042 300 H2 + Br2 2 HBr Pt (solid) 1 x 108 300 Ru (solid) 3 x 1016 500 CH3CHO CH4 + CO I2 (gas) 4 x 106 500 CH3CH3 C2H4 + H2 NO2 (gas) 1 x 109 750 (CH3)3CHO (CH3)2C=CH2+ H2O HBr (gas) 3 x 108 750 2 NO + 2 H2 N2 + 2 H2O typically by 19 – 30 kcal/mol, thus… lower the temperature where a reaction takes place 101 Catalytic Kinetics Various & significantly different from those of uncatalyzed kinetics Effect of Concentration/ Pressure Rh(111) CO 21 O 2 CO 2 Pt wire NH 3 21 N 2 32 H 2 102 Catalytic Kinetics (cont’) Temperature effect: does not obey Arrhenius law Rh(111) CO 21 O 2 CO 2 A: PCO = 2,5 x 10-8 torr B: PCO = 1,0 x 10-7 torr C: PCO = 8,0 x 10-7 torr D: PO2 = 4,0 x 10-7 torr E: PO2 = 2,5 x 10-8 torr F: PCO = 2,5 x 10-8 torr, PO2 = 2,5 x 10-8 torr Pt wire NH 3 21 N 2 32 H 2 A: PNH3 = 0,3 ; PH2 = 0,15 B: PNH3 = 0,3 ; PH2 = 0,44 C: PNH3 = 0,05; PH2 = 0,15 D: PNH3 = 0,05; PH2 = 0,45 103