Chemistry II - astchemistry
advertisement
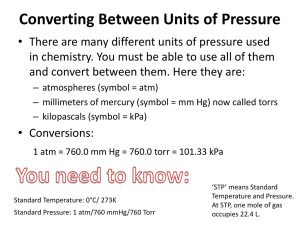
Unit 1 Gases The Nature of Gases Objectives: 1. Describe the assumption of the kinetic theory as it applies to gases. 2. Interpret gas pressure in terms of kinetic theory 3. Define the relationship between Kelvin temperature and average kinetic energy. 4. Explain why gases are easier to compress than solids or liquids. 5. Describe the 3 factors that affect gas pressure. Properties of Gases take the shape of their container low density Compressible Mixtures are homogeneous Fluids (flow) Gas pressure Results from collisions of gas particles with an object. In empty space where there are no particles, there is no pressure and is called a vacuum. Atmospheric pressure (air pressure): due to atoms and molecules in air. Barometer: used to measure atmospheric pressure. Units for measuring pressure: Pascal (Pa) Standard atmosphere (atm) Millimeters of mercury (mmHg) 1 atm = 760 mmHg = 101.3 kPa 1kpa = 1000 pa Standard pressure: 1 atm Factors affecting gas pressure Amount of gas Volume Temperature Standard temperature : 0C (273K) Converting between units of pressure A pressure gauge records a pressure of 450 kPa. What is the measurement expressed in atmospheres and millimeters of mercury? For converting to atm: 450 kpa x 1 atm = 4.4 atm 1013.kPa For converting to mmHg: 1. 450kPa x 760 mmHg = 3.4 x 103 mmHg 101.3 kPa 2. What pressure in kilopascals and in atmospheres, does a gas exert at 385 mmHg? 51.3 kPa, 0.507 atm 3. The pressure on the top of Mount Everest is 33.7 kPa. Is that pressure greater or less than 0.25atm? 33.7 kPa is greater than 0.25 atm Reaction_to_Air_Pressure_Below_Sea_Level.asf Classwork: Read pages 103-105 Do problems 1,4,5,6 Gas Laws Objectives Describe the relationships among the temperature, pressure, and volume of a gas 2. Use the gas laws to solve problems 1. Boyle’s Law : Pressure and Volume States that for a given mass of gas at constant temperature, the volume of a gas varies inversely with pressure. If pressure increases, volume decreases; if pressure decreases, volume increases. Volume could be in liters (L), mL, cm3, dm3 ,m3) 1L=1000 mL 1 cm3= 1 mL P1 x V1 = P2 x V2 P: pressure V: volume 1: initial condition 2: final condition YouTube - Self Inflating a Balloon Marshmallow Man In A Vacuum (Family & Education: Cool Experiments) Using Boyle’s Law 1. A balloon with 30.0L of helium at 103kPa rises to an altitude where the pressure is only 25.0kPa. What is the volume of the helium (at constant temperature)? P1 x V1 = P 2 x V2 2. A gas with a volume of 4.00L at a pressure of 205 kPa is allowed to expand to a volume of 12.0L. What is the pressure of the container now (at constant temperature)? P1 x V1 = P 2 x V2 Classwork: pg 121 #1 (a-c), 2, 3, 4 Charles’s Law: Temperature and Volume States that the temperature of an enclosed gas varies directly with the volume at constant pressure. As temperature increases, volume increases. V1 = V2 T1 T2 V1: initial volume V2: final volume T1: initial temperature T2: final temperature Temperature has to be in Kelvin scale. K =C + 273 Volume and Temperature As a gas is heated, it expands. This causes the density of the gas to decrease. YouTube - Balloon in liquid nitrogen Using Charles’s Law 1. A balloon inflated in a room at 24C has a volume of 4.00L . The balloon is then heated to a temperature of 58C. What is the new volume ? Since temperature increases, you expect the volume to increase. Classwork: p124 # 11, 12 (a-c), 13 Combined Gas Law Describes the relationship among the pressure, temperature and volume, when the amount of gas is constant. P1V1 = P2V2 T1 T2 Standard temperature and pressure (STP): 0C, 1 atm Useful conversions: 1L =1000 mL ; 1mL =1cm3 ; 1dm3 = 1 L Using the combined gas law: 1. The volume of a gas filled balloon is 30.0L at 313K and 153 kPa. What would the volume be at standard temperature and pressure (STP)? Classwork: p 126 #14(a), 15 (a) , 16, 17,