16 Kinetics (AHL) - slider-dpchemistry-11
advertisement

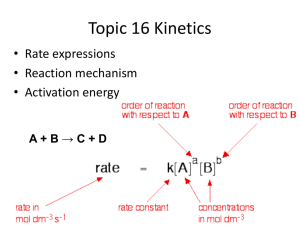
DP Chemistry R. Slider Rate Equation Recall that the rate of a reaction is a measure of the change in concentration of a reactant, R, (or product, P) over time. Notice the initial rate is measured from the graph Units for rate Δ[𝑅] 𝑅𝑎𝑡𝑒 = − Δ𝑡 Δ[𝑃] 𝑅𝑎𝑡𝑒 = Δ𝑡 The rate of disappearance of the reactants is equal to the rate of appearance of the products for a 1:1 molar ratio. This can be seen graphically above Measuring reaction rates H2(g) + I2(g) 2HI(g) The rate of reaction changes as the reaction proceeds. This can be seen by the change in the gradient of the curves on the graph. We can collect information about rate at different times by measuring the gradient at different points on the graph. Notice in the above graph, the formation of HI is initially occurring at a faster rate than the disappearance of reactants as seen by the steeper gradient. Can you explain why? Reaction Order When the concentration of a particular reactant is changed, the rate of reaction may also change This relates to reaction order which can only be determined experimentally Consider the following reaction for which we have a measured initial rate for A and B: A + B products We can alter concentrations and measure the change in rate Scenario 1: First order Doubling [A] doubles the rate of reaction. This means: Rate α [A] Or Rate = k[A] where k is the rate constant which is dependent upon temperature and use of a catalyst. This reaction is “first order with respect to A” Scenario 2: Second order Doubling [A] increases the rate 4 times. This means: Rate α [A]2 Or Rate = k[A]2 This reaction is “second order with respect to A” Reaction Order When the concentration of a particular reactant is changed, the rate of reaction may also change This relates to reaction order which can only be determined experimentally Consider the following reaction for which we have a measured initial rate: Scenario 3: Zero order Doubling [A] does not change the rate of reaction. This means: Rate α [A]0 Or Rate = k[A]0 This reaction is “zero order with respect to A” Scenario 4: Overall reaction order Doubling [A] increases the rate 4 times and doubling [B] doubles the rate. This means: Rate α [A]2[B] This is the rate Or expression for the Rate = k[A]2[B] reaction A + B products We can alter concentrations and measure the change in rate This reaction is “second order with respect to A and first order with respect to B” Overall reaction order = 2 + 1 = 3 Reaction Order Summary For a general reaction: R P, Rate α [R]m Order of Reaction with respect to reactant, R Index value Rate (value of m) equation Experimental observation Zero 0 Rate=k[R]0 No change in rate with a change in [R] One 1 Rate=k[R]1 Rate and [R] are directly proportional – as [R] is doubled or tripled, so is the rate Two 2 Rate=k[R]2 Rate is directly proportional to [R]2 – doubling [R] will increase the rate fourfold Rate Constant (k) What units? The units of the rate constant is dependent upon the overall rate expression. To determine the units for the rate constant, simply solve for k and derive the units. Source: Chemistry for use with the IB Diploma Program, Derry et. al. A small value for k is an indication of a slow rate of reaction whereas a large value is indicative of a fast reaction rate. Rate expression So, now we know the rate expression has a general form that looks something like this: For the reaction A + B products the rate expression looks like: Order of reaction with respect to B Order of reaction with respect to A Rate = k [A]m[B]n rate in mol dm-3 s-1 rate constant concentrations in mol dm-3 Be sure to practise solving problems involving this rate expression to solve for: •Rate •Rate constant •Unknown concentration Determining the rate expression Procedure: 1. Measure the initial rate for a series of reactant concentrations 2. Change one of the concentrations keeping the other constant and measure the rate again 3. Change the concentration of the one previously kept constant, and measure the initial rate again. 4. Repeat this procedure until all reactants have been changed and enough data is obtained You try: Look at the data to the right and determine the rate expression and the rate constant for a reaction that has 3 reactants, A, B and C. Experiment [A] / mol [B] / mol [C] / mol dm-3 dm-3 dm-3 Rate/ mol dm-3 s-1 1 0.1 0.1 0.1 6.2 x 10-4 2 0.1 0.2 0.1 1.2 x 10-3 3 0.1 0.1 0.2 6.2 x 10-4 4 0.2 0.1 0.2 2.5 x 10-3 Determining the rate expression Procedure: 1. Measure the initial rate for a series of reactant concentrations 2. Change one of the concentrations keeping the other constant and measure the rate again 3. Change the concentration of the one previously kept constant, and measure the initial rate again. 4. Repeat this procedure until all reactants have been changed and enough data is obtained You try: Look at the data to the right and determine the rate expression and the rate constant for a reaction that has 3 reactants, A, B and C. Exp’t 2: Doubles [B], which doubles the rate Exp’t 3: Doubles [C], which has no effect on the rate Exp’t 4: Doubles [A], which quadruples the rate Therefore, A is second order, B is first order and C is zero order and the rate expression is: Rate = k[A]2[B] (note [C]0 = 1 so is not included in the rate expression) Experiment [A] / mol [B] / mol [C] / mol dm-3 dm-3 dm-3 Rate/ mol dm-3 s-1 1 0.1 0.1 0.1 6.2 x 10-4 2 0.1 0.2 0.1 1.2 x 10-3 3 0.1 0.1 0.2 6.2 x 10-4 4 0.2 0.1 0.2 2.5 x 10-3 Solving for k, 6.2 x 10-4 = k [0.1]2[0.1]1 k = 6.2 x 10-1 dm6 mol-2 s-1 Determining reaction order Experiment We need to be able to measure the change in concentration of either a reactant or a product such as a gas being produced. Then we can change the concentration of reactants one by one to determine how they affect the reaction rate. Concentration (vol here) vs. Time graph This graph allows us to calculate instantaneous rate information. The gradient equals the rate. Rate vs. Concentration graph (not shown) Plotting rates vs. changes in concentration allows us to easily determine the order of the reaction by analysing their shape. Source: http://ibchem.com/IB/ibnotes/full/kin_htm/16.1.htm Reaction order graphs - zero Rate vs. Concentration For a zero order reaction, rate is constant with changes in concentration A products , rate = k Source: http://ibchem.com/IB/ibnotes/full/kin_htm/16.1.htm Concentration vs. Time Because the rate is constant, [A] will decrease by the same amount every second and the gradient is constant and negative (-k) Also, the time it takes for half of the reactants to disappear (1/2 life), decreases with reduced concentration Reaction order graphs - first Rate vs. Concentration For a first order reaction, the rate increases in proportion to changes in concentration A products , rate = k[A] Source: http://ibchem.com/IB/ibnotes/full/kin_htm/16.1.htm Concentration vs. Time The rate will decrease every second and the gradient will become less negative as [A] decreases Also, the 1/2 life remains constant with reduction in concentration Reaction order graphs - second Rate vs. Concentration For a second order reaction, the rate increases exponentially with increases in concentration (like y = x2) A + B products , if [A] = [B] rate = k[A]2 Concentration vs. Time The rate will decrease every second and the gradient will become less negative, but more dramatically than first order reactions Also, the 1/2 life increases with reduction in concentration Source: http://ibchem.com/IB/ibnotes/full/kin_htm/16.1.htm Exercise The reaction between NO (g) and Cl2(g) has been studied at 50 °C, recording the initial rate of formation of NOCl(g) for the initial concentrations of reactants as shown in the table . NO(g) + ½Cl2(g) NOCl(g) 1. 2. 3. 4. 5. 6. 7. [NO(g)] (mol dm-3) [Cl2(g)] (mol dm-3) Initial Rate (mol dm-3 s-1) 0.250 0.250 1.43 x 10-6 0.250 0.500 2.86 x 10-6 0.500 0.500 11.4 x 10-6 The order of reaction with respect to NO(g) is: The order of reaction with respect to Cl2(g) is: The overall order of reaction is: The value of the rate constant, k, at 50 °C is: mol-2 dm6 s-1 The rate of formation of NOCl when [NO(g)] and [Cl2(g)] are both equal to 0.110 mol dm-3 is: The rate at which NO is reacting, at the instant when Cl2 is reacting at the rate of 2.21 x 10-7 mol dm-3 s-1 is: The rate at which NOCl is forming, at the instant when Cl2 is reacting at the rate of 2.21 x 10-7 mol dm-3 s-1 is: Exercise answers The reaction between NO (g) and Cl2(g) has been studied at 50 °C, recording the initial rate of formation of NOCl(g) for the initial concentrations of reactants as shown in the table. NO(g) + ½Cl2(g) NOCl(g) 1. 2. 3. 4. 5. 6. 7. [NO(g)] (mol dm-3) [Cl2(g)] (mol dm-3) Initial Rate (mol dm-3 s-1) 0.250 0.250 1.43 x 10-6 0.250 0.500 2.86 x 10-6 0.500 0.500 11.4 x 10-6 The order of reaction with respect to NO(g) is: 2 The order of reaction with respect to Cl2(g) is: 1 The overall order of reaction is: 3 The value of the rate constant, k, at 50 °C is: 9.17 x 10-5 mol-2 dm6 s-1 The rate of formation of NOCl when [NO(g)] and [Cl2(g)] are both equal to 0.110 mol dm-3 is: 1.22 x 10-7 mol dm-3 s-1 The rate at which NO is reacting, at the instant when Cl2 is reacting at the rate of 2.21 x 10-7 mol dm-3 s-1 is: 4.42 x 10-7 mol dm-3 s-1 (half of Cl2 as seen in the reaction ratio) The rate at which NOCl is forming, at the instant when Cl2 is reacting at the rate of 2.21 x 10-7 mol dm-3 s-1 is: 4.42 x 10-7 mol dm-3 s-1 (same as the disappearance of NO in #6) Graphing Exercise Plot this data to determine the order of the reaction: 2NOBr(g) 2NO(g) + Br2 (g) Reaction Mechanisms Most reactions occur in more than one step because it is rare that more than two individual particles will simultaneously collide and successfully react. Reactions often go through intermediate species, which means a reaction may go through multiple steps before reaching the final products. These possible multi-step pathways are known as reaction mechanisms. It is important to note that there may be more than one possible mechanism. Each step in the reaction mechanism is known as the elementary step or elementary process. Source: http://www.talktalk.co.uk/reference/encyclopaedia/hutchinson/m0030471.html Molecularity This is a description of each elementary step, indicating the number of reacting particles. Quite simply: Number of reacting particles Molecularity Example Rate/order 1 Unimolecular CuCO3 CO2 + CuO k[CuCO3]/first 2 Bimolecular NO2 + CO NO + CO2 k[NO2][CO]/second 3 Termolecular 2CO + O2 2CO2 k[CO]2[O2]/third Unimolecular and bimolecular are by far the most common. Single step termolecular reactions are very rare and no examples of higher molecularity are known. Reaction Mechanism example 1 Consider this reaction: 2 NO(g) + O2 → 2 NO2 This reaction does not occur in a single step, however, but rather through two steps. Step 1: 2 NO → N2O2 Step 2: N2O2 + O2 → 2 NO2 Overall, Step 1: 2 NO → N2O2 Step 2: N2O2 + O2 → 2 NO2 Overall: 2 NO(g) + O2 → 2 NO2 Reaction Mechanism example 2 This is a reaction between 2-bromo-2methylpropane and the hydroxide ions from sodium hydroxide solution: This shows a two-step mechanism. The first step shows electrons being transferred to the Br forming two ions. This is slow due to strong bonds between the carbon andadding bromine. Notice that the two elementary steps together The second step is likely to be fast due to gives the overall balanced the strong attraction between the positive equation for the reaction carbon and negative hydroxide. Source: http://www.chemguide.co.uk/physical/basicrates/ordermech.html The slowest step is known as the rate determining step because the rate can only be as fast as the slowest step. Reaction Mechanism example 2 This is a reaction between 2-bromo-2methylpropane and the hydroxide ions from sodium hydroxide solution: Experimentally: We find that the overall rate expression is: Rate = k[(CH3)3CBr] Notice that the [OH-] has no effect on the rate. This supports the assumption that the first step is slow. If the second step were also slow, increasing the [OH-] would have an effect on the rate. Source: http://www.chemguide.co.uk/physical/basicrates/ordermech.html The rate determining step must have a rate expression that matches the rate expression of the overall reaction. The molecularity of this step is equal to the overall order of reaction. Reaction Mechanism example 3 This is a reaction between bromo-ethane and the hydroxide ions. Similar species, but different mechanism. Experimentally: We find that the overall rate expression is: Rate = k[(CH3)3CBr][OH-] Notice that in this example the [OH-] has an effect on the rate. This supports the assumption that the reaction occurs in one step. Source: http://www.chemguide.co.uk/physical/basicrates/ordermech.html The reaction occurs all at once due to the partial positive charge on the carbon atom Exercise Step 1: 2 NO → N2O2 Step 2: N2O2 + H2 → N2O + H2O Step 3: N2O + H2 → N2 + H2O For this reaction find the following: the overall balanced equation any reaction intermediates Exercise answers Step 1: 2 NO → N2O2 Step 2: N2O2 + H2 → N2O + H2O Step 3: N2O + H2 → N2 + H2O For this reaction find the following: the overall balanced equation any reaction intermediates Net Reaction: 2 NO + 2 H2 → N2 + 2 H2O To identify the reaction intermediates, look for substances that first appear on the product side of the equation, but then appear in the next step as a reactant. In this example there are two reaction intermediates - N2O2 and N2O Exercise If the reaction 2 NO 2 + F2 2 NO2F follows the mechanism, Step 1. NO2 + F2 NO2F + F (slow) Step 2. NO2 + F NO2F (fast) Work out the rate expression What is the order of the overall reaction Exercise answers If the reaction 2 NO 2 + F2 2 NO2F follows the mechanism, Step 1. NO2 + F2 NO2F + F (slow) Step 2. NO2 + F NO2F (fast) Rate = k[NO 2][F2] ..because the rate expression of the slowest step is the same as the overall rate expression. Therefore the overall order of reaction is 2. Activation Energy Recall that the activation energy, Ea, is the energy required for particles to react. We have also discussed that raising the temperature increases the number of particles that are able to react as seen in the MaxwellBoltzmann distribution curves We have also stated that the rate constant, k, is affected by temperature and catalysts. So, how can we quantify this? The rate constant is proportional to the fraction of particles that have energies ≥ Ea, represented by the shaded part of the graph. Activation Energy Mathematically, the fraction, f, of particles ≥ Ea 𝑠ℎ𝑎𝑑𝑒𝑑 𝑎𝑟𝑒𝑎 f = 𝑡𝑜𝑡𝑎𝑙 𝑎𝑟𝑒𝑎 𝑢𝑛𝑑𝑒𝑟 𝑐𝑢𝑟𝑣𝑒 and the fraction is proportional to the rate constant, k. fαk Now, all we need to do is understand this proportionality… Arrhenius Equation The fraction of particles ≥ 𝐸𝑎 𝑖𝑠 𝑓=𝑒 −𝐸𝑎 Arrhenius constant 𝑅𝑇 Where: R = gas constant 8.314 J K-1 mol-1 T = temp in K e = 2.718 (base for ln) Ea = activation energy (must be in J mol-1 to cancel) Therefore, since f α k… Activation energy 𝑘 = 𝐴𝑒 −𝐸𝑎 𝑅𝑇 rate constant Mathematical quantity (2.718) Temperature in Kelvin Gas constant (8.314 J K-1 mol-1 ) Note: the Arrhenius constant, A, is based on the probability of correct molecular orientation and frequency of collisions. It is virtually constant over a wide temperature range for a particular reaction. It is also called the frequency factor Arrhenius Equation The negative sign on the exponent means that: Activation energy Arrhenius constant • As Ea increases, k decreases • As T increases, k increases 𝑘 = 𝐴𝑒 So… −𝐸𝑎 𝑅𝑇 rate constant The rate constant will increase with increasing temperature and decrease with decreasing temperature Mathematical quantity (2.303) Temperature in Kelvin Gas constant (8.314 J K-1 mol-1 ) Arrhenius Equation – integrated form The Arrhenius Equation is also sometimes written in the so-called integrated form. This is just a rearrangement of the equation to help us to: • solve for temperature • determine Ea using a graph 𝐸𝑎 𝑙𝑛𝑘 = 𝑙𝑛𝐴 − 𝑅𝑇 Where, ln is the natural logarithm (your scientific calculator will have a button) Also, no need to memorize either of these equations. They are found in your Chemistry Data Booklet. Exercise What happens to the fraction of particles that are able to react when we raise the temperature from 20 0C to 300C, assuming the activation energy is 50 kJ mol-1? Exercise What happens to the fraction of particles that are able to react when we raise the temperature from 20 0C to 300C, assuming the activation energy is 50 kJ mol-1? 𝒆 1. 2. 𝑒 −50000𝐽 𝑚𝑜𝑙 𝑒 −𝑬𝒂 − 1 −50000𝐽 𝑚𝑜𝑙 (8.314)(293𝐾) 𝑹𝑻 = 1.21 x 10-9 − 1 (8.314)(303𝐾) = 2.38 x 10-9 This shows that an increase in 100C has effectively doubled the fraction of particles that can react. Determining Ea using a graph We can use the integrated form of the Arrhenius Equation to determine the activation energy. Recall, 𝐸𝑎 𝑙𝑛𝑘 = 𝑙𝑛𝐴 − 𝑅𝑇 This can be rewritten in the form of an equation for a straight line, y = mx + b 𝐸𝑎 𝑙𝑛𝑘 = − 𝑅 1 + 𝑙𝑛𝐴 𝑇 Where: y = ln k, y intercept = ln A 1 x=𝑇 gradient = − 𝐸𝑎 𝑅 Determining Ea using a graph So, a graph of ln k vs. 1/T will give a straight line that will allow us to determine the value of Ea for a reaction from the gradient. Now, practice some of these problems…