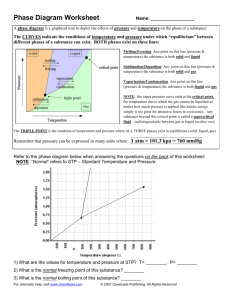
Henry`s Law - Embrace Challenge
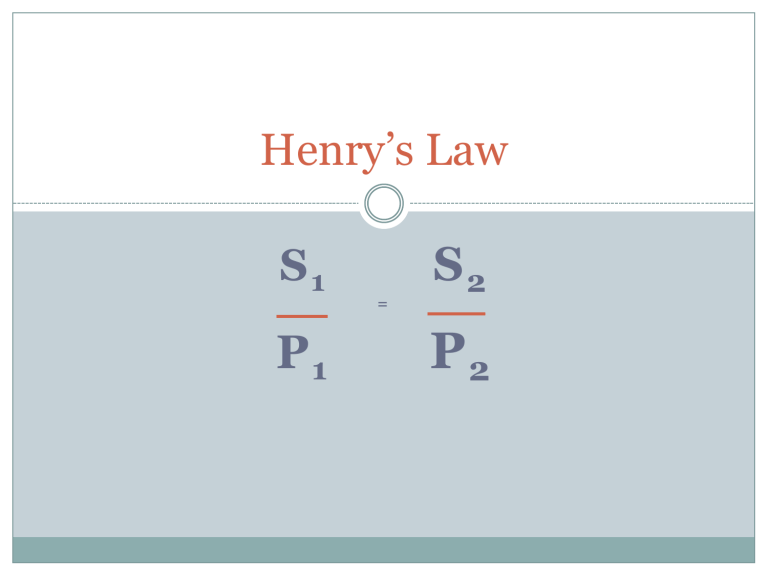
Henry’s Law
S
1
P
1
=
S
2
P
2
The question
If 0.80 g of sulfur dioxide at
10.00 atm pressure (P1) dissolves in 5.00 L of water at
25.0°C, how much of it will dissolve in 1 L of water at 9.00 atm pressure (P2) and the same temperature?
Pull out the important parts
0 . 8 0 g o f s u l f u r d i o x i d e , S O 2
I n 5 . 0 0 L
A t 2 5 ◦ C
A t 1 0 . 0 0 a t m , P 1
X g
I n 1 L
A t 2 5 ◦ C
A t 9 . 0 0 a t m , P 2
Start assembling the equation
S 1
F i r s t , C a l c u l a t e S 1
S o l u b i l i t y o f a g a s i s m e a s u r e d i n g / L
S o w e h a v e
= 0 . 8 0 g
5 . 0 0 L
= 0 . 1 6 g / L
Solve for S2
Another way of looking at the equation
S
1
S
2
=
P
1
P
2
Cross multiply to get an equation that is easy to use.
Solve for S2
Another way of looking at the equation
S
1
S
2
=
P
1
P
2
Cross multiply to get an equation that is easy to use.
S1 P2 = S2 P1
Solve for S2
Another way of looking at the equation
S1 P2 = S2 P1
We are solving for S2 so we’ll divide both sides by P1.
Yes, we could have just multiplied both sides by P2.
I am trying to think of ways to make this equation easy to remember- is it easier as
S1P2 = S2P1 or as “S1 over P1 = S2 over P2”? Use what makes your brain happy.
S1P2
P1
= S2
(0.16g/L )(9.00 atm)
10.00 atm
= 0.144 g/L
It asked for how much will dissolve in 1 L.
0.144 g
L
= x g
1 L
Hopefully the answer is obvious that 0.144 g will dissolve in 1 L.
Just for fun, what if they asked how much would dissolve in 0.75L?
0.144 g
L
= x g
0.75 L
Cross multiply to get (0.114 g) (0.75L) = (x g) (1 L)
0.0855 gL = x gL
Divide both sides by L to get 0.0855 g will dissolve in 0.75 L