Concept of the Gibbsian ensemble
Statistical Mechanics
Concept of the Gibbsian Ensemble
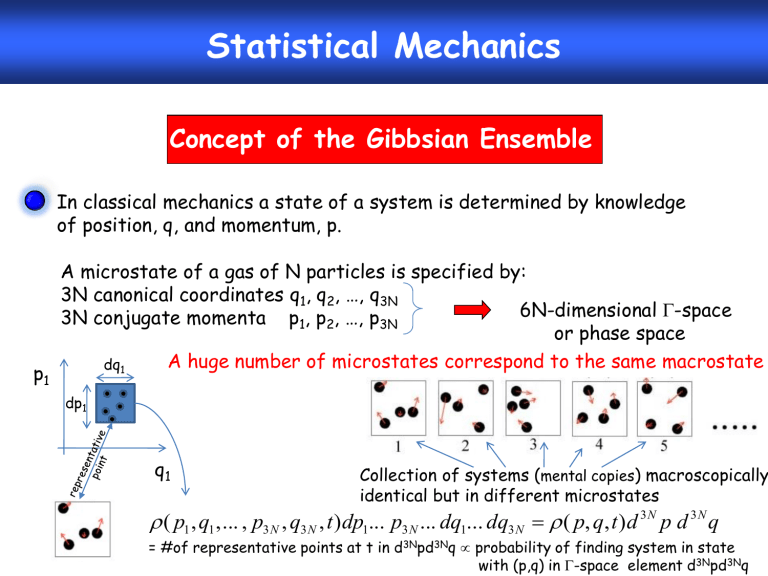
In classical mechanics a state of a system is determined by knowledge of position, q, and momentum, p.
p
1
A microstate of a gas of N particles is specified by:
3N canonical coordinates q
3N conjugate momenta p
1
1
, q
, p
2
2
, …, q
, …, p
3N
3N
6N-dimensional -space or phase space dq
1
A huge number of microstates correspond to the same macrostate dp
1
q
1
(
1
,
1
,... , p
3 N
, q
3 N
, )
Collection of systems ( mental copies ) macroscopically identical but in different microstates
1
...
p
3 N
...
dq
1
...
dq
3 N
p q t d
3 N p d
3 N q
= #of representative points at t in d 3N pd 3N q probability of finding system in state with (p,q) in -space element d 3N pd 3N q
Another way of looking at the ensemble concept: p
1 dq
1 dp
1 t
2 t
3 t
1 t
4 t
5 t
1 t
2 t
3 t
4 t
5 time q
1 time trajectory spends in d 3N pd 3N q probability of finding system in d 3N pd 3N q
Alternatively to following temporal evolution of trajectory in -space study copies 1,2,3,4,5 … at a given moment
Density in -space probability density p
1 dq
1 dp
1
Only needed when not normalized according to q
1 d
3 N p d
3 N q
( , )
1
Observed value of a dynamical quantity O(p,q)
Ensemble average
O
d
3 N p d
3 N
d
3 N p d
3 N q
( , , )
In thermal equilibrium
O
d
3 N p d
3 N
d
3 N p d
3 N q
( , )
The assumption
O
d
3 N p d
3 N
d 3 N p d 3 N q
( , )
1
T lim
T
T
0 ergodic hypothesis
Transition from classical to quantum statistics
In classical mechanics a state of a system is determined by knowledge of position, q, and momentum, p.
Dynamic evolution given by : p i
H
q i
, q i
H
p i trajectory in -space
( , , )
3 N 3 N p q t d p d q =probability that a system’s phase point (p,q) is in with
3 N 3 N d p d q
( , , )
3 N p d
3 N q
1
In quantum mechanics a state of a system is determined by knowledge of the wave function .
Thermodynamic description is given in terms of microstates that are the system’s energy eigenstates determined from
H
( ,
2
, ..., r
N
)
E
r r
2
, ..., r
N
)
Eigenfunctions labels set of quantum number
Eigenenergies
classical
( , , )
3 N 3 N p q t d p d q with
=probability that a system’s phase point (p,q) is in
3 N 3 N d p d q
( , , )
3 N p d
3 N q
1
quantum
=probability of system being in state label by with
1
X
3 N p d 3 N q
X
X
Note: Later we will discuss in more detail the transition from the classical density function to the quantum mechanical density matrix