week 6,7
advertisement

The properties of mixtures
자연과학대학 화학과
박영동 교수
Chapter 6 The properties of mixtures
6.1 The thermodynamic description of mixtures
6.1.1
6.1.2
6.1.3
6.1.4
6.1.5
Partial molar properties
Spontaneous mixing
Ideal solutions
Ideal dilute solutions
Real solutions: activities
6.2 Colligative properties
6.2.6 The modification of boiling and freezing points
6.2.7 Osmosis
6.3 Phase diagrams of mixtures
6.3.8 Mixtures of volatile liquids
6.3.9 Liquid-liquid phase diagrams
6.3.10 Liquid-solid phase diagrams
6.3.11 The Nernst distribution law
The partial molar volumes of
water and ethanol at 25°C.
𝜕𝑉
𝑉𝑗 =(𝜕𝑛 )𝑝,𝑇,𝑛′
𝑗
partial molar volume
At 25°C, the density of a 50 per cent by mass
ethanol/water solution is 0.914 g cm-3. Given that the
partial molar volume of water in the solution is 17.4
cm3 mol-1, what is the partial molar volume of the
ethanol?
partial molar Gibbs energy, GJ
Chap. 5
Pressure dependence of G
G = H – TS
dG = dH – TdS – SdT = Vdp - SdT
𝜕𝐺
( ) =
𝜕𝑝 𝑇
V
For liquid or solid, ΔG = VΔp
For vapor, ΔG = ∫Vdp
= nRT ∫(1/p)dp
=nRT ln(pf/pi)
ΔGm = RT ln(pf/pi)
ch05f01
The Gibbs energy of mixing of two perfect gases of
two liquids that form an ideal solution.
Chap. 4
The entropy change with
isothermal expansion
S
1
2
dq
T
1
2
1
2
p
ch04f04
1
T
dV
2
nR
V
V
p
nR ln 2 nR ln 2
V1
p1
1
T
dw
1
dV
The entropy of mixing of two perfect gases of two
liquids that form an ideal solution.
Raoult's law:
pA =xApA*
Raoult’s law
The partial vapour pressure
of a substance in a liquid
mixture is proportional to its
mole fraction in the mixture
and its vapour pressure when
pure:
Figure 6.6 The partial vapour pressures of the two
components of an ideal binary mixture are
proportional to the mole fractions of the components
in the liquid. The total pressure of the vapour is the
sum of the two partial vapour pressures.
certain composition makes
the solution more volatile
CS2 is more volatile
Raoult’s law: for Ideal solution, esp. for solvent
The partial vapour pressure
of a substance in a liquid
mixture is proportional to its
mole fraction in the mixture
and its vapour pressure when
pure:
Figure 6.6 The partial vapour pressures of the two
components of an ideal binary mixture are
proportional to the mole fractions of the components
in the liquid. The total pressure of the vapour is the
sum of the two partial vapour pressures.
chemical potential of a solvent A present in solution
at a mole fraction xA is
At equilibrium, chemical potential of any
given component is same everywhere.
Henry’s law, for ideal solutes
The experimental partial vapour pressures
of a mixture of trichloromethane, CHCl3
(C), and propanone, CH3COCH3 (acetone,
A),
The chemical potential of the solute has its
standard value when the molar concentration
of the solute is 1 mol dm−3 (that is, ).
G = H – TS
dG = dH – TdS – SdT = Vdp – SdT
dμ = Vmdp – SmdT
μs = μ*– Sm(s) dT
μl = μ*– Sm(l) dT + RT ln xA
{Sm(l) - Sm(s) }ΔT = RT ln xA
-Sm(s)ΔT
-Sm(l)ΔT
μ*
RT ln xA
μ*+ RT ln xA
μg = μ*– Sm(g) dT
μl = μ*– Sm(l) dT + RT ln xA
μ*
{Sm(l) - Sm(g) }ΔT = RT ln xA
{Sm(l) - Sm(g) }ΔT = RT ln xA
Sm(g)ΔT
μ*+ RT ln xA
Sm(l)ΔT
μA(xA=1, p)
μA(xA, p+Π)
μA(xA=1, p) = μA(xA, p+Π)
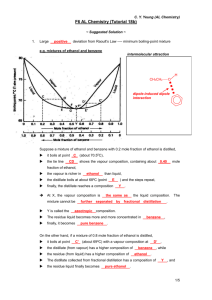
Understanding fractional distillation
pA = xA pA *= a pA *
pB = xB pB * = (1-a) pB *
a'= pA /(pA + pB) = a pA * /(a pA * + (1-a) pB *)
= a pA * /(pB * + a(pA *- pB * ))
Liquid-Vapor Composition and
fractional distillation
a'= yA = mole fraction in vapor
a'= pA /(pA + pB)
= a pA * /(a pA * + (1-a) pB *)
= a pA * /(pB * + a(pA *- pB * ))
a'= aX/(1+a(X-1))
where X = (pA * / pB *)
a= xA = mole fraction in liquid
B: less volatile substance
나오는 것은 A, 남는 것은 B
A: more volatile substance
lever rule and phase diagram
low-boiling(positive) azeotrope
repeated distillation can never produce a
distillate that is richer in constituent X
than the azeotrope
끓어 나오는 것
은 Azeotrope,
남는 것은 A나 B
공비(共沸) 혼합물
high-boiling(negative) azeotrope
끓어 나오는
것은 A나 B이
지만, 남은 것
은 Azeotrope