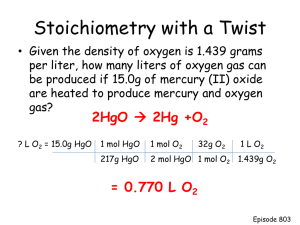Chapter 3: Stoichiometry
advertisement

Chapter 3: Stoichiometry • Stoichiometry - The study of quantities of materials consumed and produced in chemical reactions. • In order to understand stoichiometry, we must first understand atomic masses Atomic Masses • Elements occur in nature as mixtures of isotopes • Carbon = 98.89% 12C 1.11% 13C <0.01% 14C Carbon atomic mass = 12.01 amu Try this… Naturally occurring lithium is: 7.42% 6Li (6.015 amu) 92.58% 7Li (7.016 amu) • Find the average atomic mass of lithium. 7.42(6.015 ) 92.58 7.016 100 6 .9 4 1a m u How about this one? Fill in the blanks. Isotope 28 Si 29 Si 32 Si Mass (amu) Abundance 27.98 29.97 4.70% 3.09% The mole • A unit to count the number of molecules Dozen = 12 Pair = 2 • 1 mol = 6.022 x 1023 Samples of 1 mole of Cu, Al, Fe, S, I, and Hg eggs Molar mass is the mass of 1 mole of shoes in grams marbles atoms 1 mole 12C atoms = 6.022 x 1023 atoms = 12.00 g 1 12C atom = 12.00 amu 1 mole 12C atoms = 12.00 g 12C 1 mole lithium atoms = 6.941 g of Li For any element atomic mass (amu) = molar mass (grams) Try this! Which of the following 100.0 g samples contains the greatest number of atoms? a) Magnesium b) Zinc c) Silver Rank the following according to number of atoms (greatest to least): a) 100.0 g of silver b) 62.0 g of zinc c) 21.0 g of magnesium Molar mass • My expectation is that you do NOT need a refresher in molar mass! So try this!!! • What is the molar mass of isopentyl acetate (C7H14O2)? If 1 g of C7H14O2 is released in a bee sting, how many molecules are released? • HINT: – g = 1x10-6g – g mol molecules • How many atoms of carbon are present? How many C atoms in bee sting? • HINT: each molecule of isopentyl acetate has 7 atoms of carbon Percent Composition or Mass % • There are two ways to describe the composition of a compound. – The number of constituents atoms – The percent of each of its elements (by mass) isopentyl acetate (C7H14O2) • Let’s look at mass percent of an element m ass% n m olar m ass of elem ent 100% m olar m ass of com poun d • Find the mass % of C m a ss% 7 1 2 .0 1 100% 1 3 0 .1 8 m a ss% o f C 6 4 .5 8 % Try this one. Consider separate 100.0 gram samples of each of the following: H2O, N2O, C3H6O2, CO2 • Rank them from highest to lowest percent oxygen by mass. Formulas • molecular formula = (empirical formula)n [n = integer] • molecular formula = C6H6 = (CH)6 • empirical formula = CH – The lowest ratio of atoms in a molecule (mole ratio) Working backwards • From the percent composition, you can determine the empirical formula. Determine the empirical formula of a compound that has the following percent composition by mass: K 24.75%, Mn 34.77% , O 40.51% Assume 100 g sample of the compound n K 24.75g n M n 34.77g 1m ol K 0.6330m olK 39.10g K 1m ol M n 0.6329m olM n 54.94g M n n O 40.51g 1m ol O 16.00g O 2.532m ol O nK = 0.6330, nMn = 0.6329, nO = 2.532 K 0 .6 3 3 0 1 .0 0 .6 3 2 9 Mn 0 .6 3 2 9 1 .0 0 .6 3 2 9 O 2 .5 3 2 4 .0 0 .6 3 2 9 KMnO4 This one has a twist! • A compound is made of only sulfur and nitrogen. It is 69.6% S by mass. Its molar mass is 184 g/mol. What is its empirical and molecular formula? How to “Read” Chemical Equations 2 Mg + O2 2 MgO 2 atoms Mg + 1 molecule O2 makes 2 formula units MgO 2 moles Mg + 1 mole O2 makes 2 moles MgO 48.6 grams Mg + 32.0 grams O2 makes 80.6 g MgO NOT 2 grams Mg + 1 gram O2 makes 2 g MgO Things to know about equations • They are sentences • They describe what happens in a chemical reaction • Reactants Products • They should be balanced • They have the same number of each kind of atoms on both sides Try this Chromium compounds exhibit a variety of bright colors. When solid ammonium dichromate (NH4)2Cr2O7, a vivid orange compound is ignited a spectacular reaction occurs. Assume that the products are chromium (III) oxide, nitrogen gas, and water vapor. Write and balance the equation. And this one • At 1000oC, ammonia gas, (NH3) reacts with oxygen gas to form gaseous nitric oxide, and water vapor. This reaction is the first step in the commercial production of nitric acid by the Ostwald process. Write and balance the equation for this reaction. Stoichiometry How much do you remember? • Methanol burns in air according to the equation 2CH3OH + 3O2 2CO2 + 4H2O If 209 g of methanol are used up in the combustion, what mass of water is produced? A good FRQ for class. • Methane (CH4) reacts with the oxygen in the air to produce carbon dioxide and water. • Ammonia (NH3) reacts with the oxygen in the air to produce nitrogen monoxide and water. • What mass of ammonia would produce the same amount of water as 1.00 g of methane reacting with excess oxygen? 1.4g ammonia Limiting Reactant • Reactant that determines the amount of product formed. • The one you run out of first. • Makes the least product. • Three methods to solve: – Ratio method shown in text – Have versus need – Do 2 stoichiometry problems to find the amount of product. The reactant that produces the LEAST amount is the limiting reagent. Let’s try together! • Nitrogen gas can be prepared by passing gaseous ammonia over solid copper(II) oxide at high temperatures. The other products of the reaction are solid copper and water vapor. If a sample containing 18.1g of NH3 is reacted with 90.4g of CuO, which is the limiting reactant? How many grams of nitrogen gas will be formed? • Have versus need method: g NH3mol NH3 mol CuO neededg CuO needed 2NH3 + 3CuO N2 +3Cu + 3H2O 1 8 .1g N H 3 1m o lN H 3 1 7 .0 4 g N H 3 3m o l C u O 2m o lN H 3 7 9 .5 5 g C u O 127g CuO needed 1m o l C u O • The problem says the we HAVE 90.4g of CuO. • Using ALL of the NH3 given we NEED 127g of CuO; therefore, the CuO is the limiting reactant since we don’t have enough of CuO • You can do this starting with the CuO as well. Let’s double check our work. 2NH3 + 3CuO N2 +3CuO + 3H2O 9 0 .4 g C u O 1m o l C u O 7 9 .5 5 g C u O 2m o lN H 3 3m o l C u O 1 7 .0 4 g N H 3 1m o lN H 3 1 2 .9 g N H 3 n e e d e d • The problem says the we HAVE 18.1g of NH3. • Using ALL of the CuO given we NEED 12.9g of NH3; therefore, the CuO is the limiting reactant since we have an excess of NH3. • There is an excess of 5.2g of NH3 2NH3 + 3CuO N2 +3CuO + 3H2O • Do the stoichiometry problem twice. The reactant that gives you the LEAST amount of product is the limiting reactant. 9 0 .4 g C u O 1 8 .1g N H 3 1m o l C u O 1m o lN 2 2 8 .0 2g N 2 7 9 .5 5C u O 3m o l C u O 1m o lN 2 1m o lN H 3 1m o lN 2 2 8 .0 2g N 2 1 7 .0 4 g N H 3 2m o lN H 3 1m o lN 2 1 0 .6 g N 2 m a d e 1 4 .9 g N 2 m a d e • The CuO is the limiting reactant. This confirms our results from the have versus need method. Try one on your own. • When 124 grams of aluminum are reacted with 601 grams of iron(III) oxide, aluminum oxide and solid iron are produced. Calculate the mass of aluminum oxide formed. Excess Reactant (reagent) • The reactant you don’t run out of. • The amount of stuff you make is the yield. • The theoretical yield is the amount you would make if everything went perfect. • The actual yield is what you make in the lab. Percent Yield % yie ld a ctu a l 100% th e o re tica l OR % yie ld w h a t yo u g o t w h a t yo u co u ld h a v e 100% Try this Methanol is the simplest alcohol. It is used as a fuel in race cars and is a potential replacement for gasoline. Suppose 68.5kg CO(g) is reacted with 8.6kg H2(g). Calculate the theoretical yield of methanol. If 3.57x104g CH3OH is actually produced, what is the percent yield?