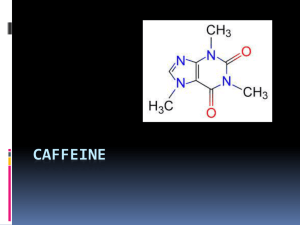
Using C1V1=C2V2
advertisement

Using C1V1=C2V2 What do all those things stand for? C1= concentration 1 V1= volume 1 C2= concentration 2 V2= volume 2 So when do we use it? We need to make solutions from stock solutions. Example We need to make a 1x concentration of stain but it comes as a 10x concentration. We know we need 250 ml of the 1 x solution for the experiment. How do we figure it out? Fill in what we know: C1- 1ox V1-? C2- 1x V2- 250 ml Now set it up 10(V1)=(1)(250ml) Now do the math! 10(V1)=(1)(250ml) Now do the math! 10(V1)=(1)(250ml) 10v1=250 ml 10 10 Now do the math! 10(V1)=(1)(250ml) 10v1=250 ml 10 10 V1= 25 mL So now how do we make it… 1st we need to figure out the difference between how much we want at the end and how much of our stock we are adding so… 250 ml- 25 ml= 225 ml (V2) (V1) The Steps Obtain 25 ml of Stock Solution and bring to volume by adding 225 ml of water. Partner Practice Problems