lecture 5 ligand substitution
advertisement

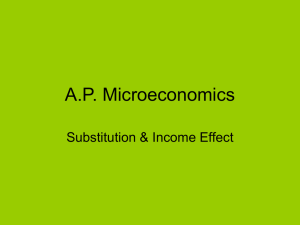
LECTURE 5: ORGANOMETALLIC REACTIONS I LIGAND SUBSTITUTION LIGAND SUBSTITUTION Cl Cl 2Pd Cl Cl C 2H 4 Cl Cl H 2O Pd Cl Cl H 2O Pd Cl OH- P d (0 ) + H + + H 2 O + 2 C l- Cl H 2O OH Cl H 2O - 2 e (C u C l 2 C u C l) Cl - C l- Pd Pd OH Cl Cl H 2O H Pd C H 3C H O -H e lim C lCl H 2O O " -H e lim " Pd H OH OH Cl H 2O Pd in s Cl H 2O Pd H LIGAND SUBSTITUTION Basic premise about metal-catalyzed reactions: • Reactions happen in the coordination sphere of the metal • Reactants (substrates) come in, react, and leave again • Binding or dissociation of a ligand is often the slow, rate-determining step Ligand Substitution 4 LIGAND SUBSTITUTION This premise is not always correct, but it applies in the vast majority of cases. Notable exceptions: • Electron-transfer reactions • Activation of a single substrate for external attack – peroxy-acids for olefin epoxidation – CO and olefins for nucleophilic attack 2 MAIN MECHANISTIC PATHWAYS ASSOCIATIVE (A): LnML’ + L’’ LnML’L’’ slow DISSOCIATIVE (D): L’’ LnML’ slow LnM + L’ fast LnML’’ fast LnML’’ + L’ DISSOCIATIVE LIGAND SUBSTITUTION Example: L nM CO 18 e L nM + CO 16 e L' L nM L' 18 e Factors influencing ease of dissociation: • 1st row < 2nd row > 3rd row • d8-ML5 > d10-ML4 > d6-ML6 • stable ligands (CO, olefins, Cl-) dissociate easily (as opposed to e.g. CH3, Cp). Ligand Substitution 7 DISSOCIATIVE SUBSTITUTION at ML6 16-e ML5 complexes are usually fluxional; the reaction proceeds with partial inversion, partial retention of stereochemistry. or 1 6 -e 1 8 -e oct SP Ligand Substitution d is to rte d TBP 8 ASSOCIATIVE LIGAND SUBSTITUTION Example: L nM 16 e L' - L L nM L' L n -1 M 18 e 16 e (N H 3 ) 2 P tC l 2 L' B r- C ls lo w H 2O - C l Sometimes the solvent is involved. Reactivity of cis-platin: (N H 3 ) 2 P t(C l)(B r) B rfa s t - H 2 O (N H 3 ) 2 P t(C l)(H 2 O ) + - C l- N u c le o B a s e fa s t s lo w - H 2O (N H 3 ) 2 P t(C l)(N B ) + Ligand Substitution 9 trans influence and trans effect • In square planar complexes, some ligands direct substitution to a position trans to themselves. • When reaction is controlled by factors influencing the ground state energy of the complex – trans influence • Reaction is controlled by factors affecting the transition state energy. trans influence Ligands that form strong bonds or tend to weaken the metal ligand bond trans to the metal. In the ground state this property is called the trans influence. H- > PR3 > SCN- > I-, CH3-, CO, CN- > Br- > Cl- > NH3 > OH- trans kinetic effect • Tendency of certain ligands to direct incoming groups to trans position with reactions under kinetic control. C2H4, CO > CN- > NO2- > SCN- > I- > Br- > Cl- > NH3> OH- overall trans effect CO, CN-, NO C2H4 > PR3, H- > CH3-, S=C(NH2)2 > PhNO2- SCN-, I-, > Br- > Cl- > Py, NH3, OH- H2O Exercise 7.1 ASSOCIATIVE LIGAND SUBSTITUTION Example: L nM 16 e L' - L L nM L' L n -1 M 18 e L' 16 e Sometimes the solvent is involved. Reactivity of cis-platin: Ligand Substitution 15 rate Rate = ks[ML4] = k1 [ML4][Y] Mechanism: Square pyramidal – trigonal bipyramid – with retention of configuration. Associative substitution with 18 e systems • Can occur if the metal can delocalize a pair of electrons onto one of its ligands LIGAND REARRANGEMENT Several ligands can switch between n-e and (n-2)-e situations, thus enabling associative reactions of an apparently saturated complex: M N O M N O 3 -e 1 -e O CO M R M M M 5 -e 3 -e R (1 + 2 )-e 1 -e Ligand Substitution 18 DISSOCIATIVE LIGAND SUBSTITUTION Example: L nM CO 18 e L nM + CO 16 e L' L nM L' 18 e Rate = k [ML6] Ligand Substitution 19 Rate of substitution of Ligands Rate of substitutions of a particular ligand is a function of ligand type. Ligands that are nuetral in their free state dissociate rather easily. Redox-induced ligand substitution Unlike 18-e complexes, 17-e and 19-e complexes are labile. Oxidation and reduction can induce rapid ligand substitution. L' L nM - e- + 1 7 -e L nM L' + 1 9 -e L nM 1 8 -e + e- L nM 1 9 -e L n -1 M - + L 1 7 -e Ligand Substitution 22 Redox-induced ligand substitution F e(C O )4L F e(C O )5 CO F e(C O )4 F e(C O )5 F e(C O )4L L Initiation by added reductant. Sometimes, radical abstraction produces a 17-e species Ligand Substitution 23 Photochemical ligand substitution Visible light can excite an electron from an M-L bonding orbital to an M-L antibonding orbital (Ligand Field transition, LF). This often results in fast ligand dissociation. M (C O )6 d h d Ligand Substitution 24