1 X 10
advertisement

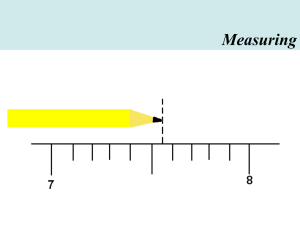
Welcome to Physics • CALCULATOR!!!!! • Homework Policy • I will provide you with a lab notebook Measuring Measuring Measuring MKS system MKS – Meter-KilogramSecond Meter • Originally 1/10,000,000 the distance from the equator to either pole • Distance light travels in 1/299,792,458th of a second (c is constant everywhere in the universe) Historical Pt-Ir meter bars Kilogram • Pt-Ir cylinder at the International Bureau of Weights and Measures. • Rest mass of 6.022 X 10 U.S. National Kilogram (NIST) Second • 1/86,400 of a mean solar day • Defined in terms of frequency of radiation emitted by a cesium isotope Cesium Fountain Clock at NIST Giga (G) = 109 Mega (M) = 106 kilo (k) = 103 hecto (h) = 102 deka (da) = 101 deci (d) = 10-1 centi (c) = 10-2 milli (m) = 10-3 micro (m) = 10-6 nano (n) = 10-9 pico (p) = 10-12 The Metric System Examples: 1 km = 1 X 103 m 450 km = 450 X 103 m = 4.5 X 105 m 45 uF = 45 X 10-6 F = 4.5 X 10-5 F The Metric System Examples: 600 nm 0.0055 Gs 5677 kg =?m =?s =?g The Metric System Examples: 600 nm 0.0055 Gs 0.000567 kg = 6 X 10-7 m = 5.5 X 106 s = 5.67 X 10-1 g Metric Example One: How many meters is 55 cm? Step 1: Step 2: Step 3: Step 4: 55 cm 55 cm X m cm 55 cm X 1 X 10-2 m 1 cm 55 cm X 1 X 10-2 m = 0.55 m 1 cm ALWAYS include this zero Metric Example 2: How many milliters is 0.0250 liters? Step 1: Step 2: Step 3: Step 4: 0.0250 L 0.0250 L mL L 0.0250 L 1 mL 1 X 10-3 L 0.0250 L 1 mL = 25.0 mL 1 X 10-3 L Metric Example 3 How many kilograms is 13405 mg? 13405 mg 1 X 10-3 g 1 mg 13405 mg 1 X 10-3 g 1 kg 1 mg 1 X 103 g 13405 mg 1 X 10-3 g 1 kg = 0.013405 kg 1 mg 1 X 103 g Metric Example 4 How many milliseconds is 0.0450 hectoseconds? (Ans: 4500 ms) Metric Practice Examples 4658 cm = 635 cm = 553 ms = 0.0023 kL = 0.468 cm = 7200 cs = 3498 s = ? km ? dam ? ds ? mL ? mm ? das ? hours Metric Practice Examples 4658 cm = 635 cm = 553 ms = 0.0023 kL = 0.468 cm = 7200 cs = 3498 s = 0.04658 km 0.635 dam 5.53 ds 2300 mL 4.68 mm 7.2 das 0.9717 hours Metric Example 5 How many square meters is 685 cm2? 685 cm2 1 X 10-2 m 1 cm 685 cm2 1 X 10-2 m 1 X 10-2 m 1 cm 1 cm 685 cm2 1 X 10-2 m 1 X 10-2 m = 0.0685 m2 1 cm 1 cm Metric Example 6 How many square decimeters is 0.250 m2? 0.250 m2 1 dm 1X10-1 m 0.250 m2 1 dm 1 dm 1X10-1 m 1X10-1 m 0.250 m2 1 dm 1 dm = 25.0 dm2 1X10-1 m 1X10-1 m Metric Example 7 How many cubic centimeters (cm3) is 0.00453 m3? (Ans: 4520 cm3) Challenge Problem The tallest building in the world is in Taiwan, Taipei 101. It is 509 meters tall. How many feet is that? (1 cm = 2.54 inches) http://en.wikipedia.org/wiki/Image:Taipei_ 101_International_Finadncial_Center.jpg Metric Example 9 Convert 22 miles/hour to m/s. This problem will require us to do two things: convert the distances and convert the time. 22 miles 1 hr 1.61 km 1X103m 1 hr 1 min = 9.8 m/s 1.00 mile 1 km 60 min 60 s Metric Example 10 Convert 200 cm/s to miles/hour. 200 cm 1s 1X10-2 m 1 km 1.00 mile 60 s 1 cm 1X103 m 1.61 km 1 min 60 min 1 hr = 4.47 miles/hr Metric Practice Examples 55 mi/hr 55 mi/hr 65 miles/hr 400 cm/s km/hr meters/min meters/s miles/hr Metric Practice Examples 55 mi/hr 55 mi/hr 65 miles/hr 400 cm/s 89 km/hr 1476 meters/min 29.1 meters/s 8.94 miles/hr Accuracy and Precision • Accuracy – how close the average of a set of measurements is to the true value – Measured using Percent Error • Precision – How close a set of measured values are to one another – Measured using Range Accuracy and Precision Students did trials to measure the density of a metal. The accepted density is 7.2 g/cm3. Were they accurate or precise? Set 1 Set 2 Set 3 7.21 7.25 7.18 6.40 7.90 7.30 6.45 6.52 6.48 Error Analysis Percent Error – Measure of accuracy % Error = Experimental – Accepted X 100 Accepted NOTE: The “Experimental” value is always the average of all your trials in an experiment Error Analysis: Example 1 A student measures the density of a sample of copper and determines it to be 8.75 g/mL. The accepted value is 8.96 g/mL. Calculate the percent error. Error Analysis: Example 2 A student measures the melting point of a sample of beryllium at 667 oC. The accepted value is 649 oC. Calculate the percent error. Error Analysis: Range Range - Measure of precision Range = highest trial – lowest trial Example 1 A student measures the melting point of a sample of beryllium and does four trials. The trials result in melting points of 667 oC, 645 oC, 670 oC, 655 oC. Calculate the range and comment on precision. Error Analysis: Range Example 2 A student measures the density of a sample of lead and does four trials. The trials result in densities of 11.3, 10.5, 11.9, 10.8 g/cm3. Calculate the range and comment on precision. Error Analysis Example 3 Using the numbers in the previous example, calculate percent error. The accepted density of lead is 11.4 g/cm3.