File
advertisement
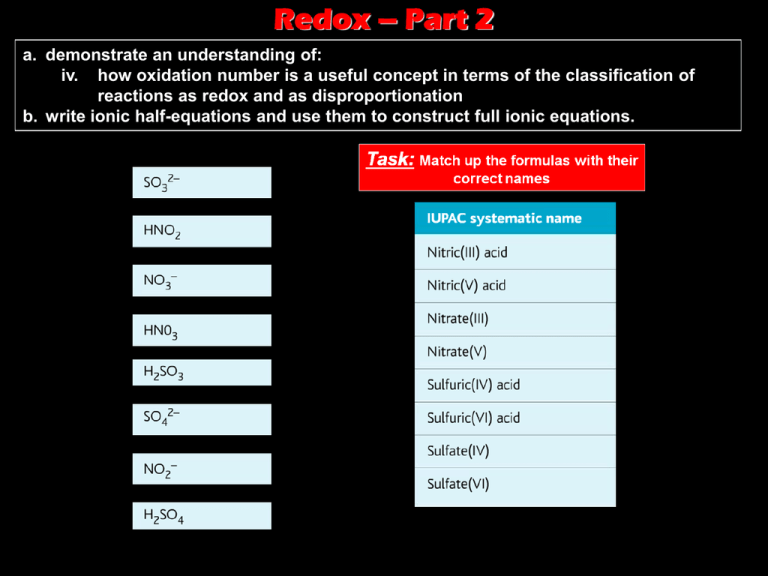
Redox – Part 2 a. demonstrate an understanding of: iv. how oxidation number is a useful concept in terms of the classification of reactions as redox and as disproportionation b. write ionic half-equations and use them to construct full ionic equations. Task: Match up the formulas with their correct names Naming Compounds • Some elements can have more than one oxidation number. • So to avoid confusion when naming the compounds they form, its helpful to use these numbers in their names. Naming Compounds What are the missing oxidation numbers here for Manganese and Chromium, respectively? ? ? Task Answer – sometimes, instead of using the systematic name, scientists still use the traditional names for some substances. Redox and Oxidation Numbers • An increase in oxidation number means oxidation has occurred. • While a decrease in oxidation number means reduction has occurred. What would be • E.g. 0 +1 -2 +1 -1 0 Therefore, chlorine has been reduced, and sulphur has been oxidised. the oxidation numbers for each of the species in this reaction? Redox and Oxidation Numbers • Another example is the Thermite Reaction (click here to see it in action!!) +3 0 -2 0 +3 • But what is oxidised and what is reduced? Therefore, iron has been reduced, and aluminium has been oxidised. -2 Disproportionation • Is a type of reaction in which a substances both oxidised and reduced in the same reaction. E.g. the breakdown of hydrogen peroxide: Disproportionation – another example • The reaction of copper(I) oxide with dilute sulphuric acid: • Study the equation carefully – what disproportionates? • The answer is copper (Cu) which gets oxidised from +1 to +2 and also reduced from +1 to zero, in the same reaction. • Other examples of such reactions include reactions of chlorine with water, and chlorine with hot sodium hydroxide solution. Questions Answers Oxidation States • Some elements show a range of oxidation states. • One colourful example is the reduction of vanadium (V) to vanadium (II) through sucessive oxidation number. Vanadium – its different oxidation states Ammonium Vanadate (V) (white solid) can be added to dilute HCl to give the orange Dioxovanadium (V) ion: VO3- + 2H+ VO2+ + H2O +5 +5 If granulated zinc is now added it reduces the vanadium over a period of several minutes and gives several colour changes: Blue [VO(H2O)5]2+ Vanadium (IV) Green [VCl2(H2O)4] + Vanadium (III) Violet [V(H2O)6]2+ Vanadium (II) Common Oxidising Agents • What is an oxidising agent? – A species that reacts by oxidising something else and getting reduced itself. • So, as we will see, electrons in the half equations are always on the left-hand side of the equation, as oxidising agents gain electrons. More Oxidising Agents More Oxidising Agents Common Reducing Agents • What is an reducing agent? – A species that reacts by reducing something else and getting oxidised itself. • So, this time, electrons in the half equations are always on the right-hand side of the equation, as reducing agents loose electrons. More Reducing Agents More Reducing Agents Writing balanced equations from ionic half equations - Worked Example… Firstly, write down the half equations you need. Worked Example…..cont…. X5 Worked Example…..cont…. Notice, that in adding the two equations together The electrons are cancelled out and not Included in the final equation Worked Example 2… Again, start with the half equations Have a go before you turn to the next slide for the answer Worked Example 3… Again, start with the half equations ? Or X Ionic to Molecular Equation • To turn an ionic equation to a molecular equation, you would simply add the spectator ions. • Eg. Reaction of potassium manganate (VII) solution with iron (II) sulphate solution in the presence of dilute sulphuric acid: Ionic equation – which you work out Add in spectator ions by using clues In question molecular equation Questions Answers