Postulate 1
advertisement
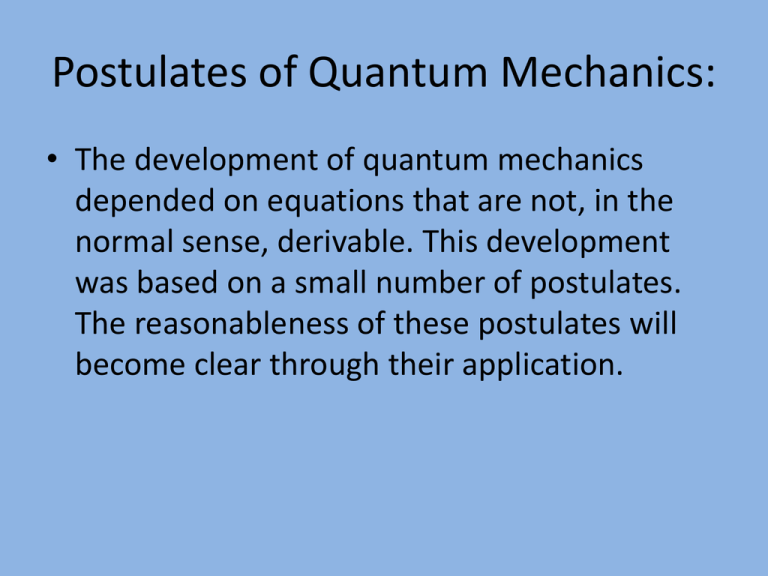
Postulates of Quantum Mechanics: • The development of quantum mechanics depended on equations that are not, in the normal sense, derivable. This development was based on a small number of postulates. The reasonableness of these postulates will become clear through their application. Quantum Mechanics – Postulates: • Postulate 1: A quantum mechanical system or particle can be completely described using a wave function, ψ. (Wave functions were introduced briefly in Chemistry 1050). • In different examples the problems of interest will have varying dimensionality. Correspondingly, one sees wave functions described in terms of one or more coordinates/variables. Postulate 1 – Dimensionality: • • • • • Possible “forms” of wave functions: One dimensional problems: ψ(x), ψ(r), ψ(θ) Two dimensional problems: ψ(x,y) etc. Three dimensional problems: ψ(x,y,z), ψ(r,θ,φ) If the time dependant evolution of the system needs to be treated (or, appears interesting!) the wave functions will have the form ψ(x,y,z, t) or ψ(r,θ,φ,t). Postulate 1 – Wave function Properties: • The wave function ψ(x,y,z) (for example) must be continuous, single valued (not a new requirement!) and square integrable. Both real and imaginary wave functions are encountered. Thus, if ψ(x) is imaginary, for example, eimx , where m is an integer, then we will need the complex conjugate wave function ψ*(x) = e-imx. Quantum Mechanics and Probability: • No fisherman would expect that the probability of finding a salmon would be the same in every pool on a river. It is perhaps not entirely surprising that, for a particular molecule, the probability of finding an e- will be different depending on what part of the molecule is considered. Probability information for quantum mechanical systems follows from the form of the wave function, ψ. Probability and Classical Waves: • Imagine making a wave by throwing a rock into a swimming pool. If one considers only the water molecules displaced upwards from their equilibrium position then the probability of finding an upward displaced water molecule is highest where the waves have the highest positive amplitude. Similar considerations apply to downward displaced water molecules. Probability and Quantum Mechanics: Probability and QM – continued: Probability and QM – continued: Probability and Normalized ψ’s: • If the integrals discussed are equal to one we say that our wave function is normalized. A similar idea may have been encountered previously when you considered the dot product of two vectors. If the integral is not equal to one we can normalize the wave function after first evaluating an integral of the form discussed. Normalizing Wave Functions: Normalized and Un-normalized Wave Functions: • The use of un-normalized wave functions in quantum mechanical problems does enable us to calculate from the Schrödinger equation the correct values for eigenvalues which specify energy and momentum values. However, the use of normalized wave functions does afford both mathematical and conceptual advantages. In particular, “probabilities” are more readily calculated. Class Example – Simple Normalization: • The simplest normalizations involve a one dimensional integration. Normalizations involving imaginary wave functions can appear ominous – until one tries them! • Example: Normalize the wave function eimθ where r is a scalar (it could be a quantum number!) and the allowed θ values are defined by 0 ≤ θ ≤ 2π. Simple Normalization – continued: • Hint: in the example to be considered the trial and possibly un-normalized wave function is • ψTrial(θ) = eimθ • This wave function will “reappear” when we move to discussions of the “particle on the ring” and angular momentum. ( Sadly (?), for Canadians, there are no known wave functions for the “particle (puck) on a rink”!) Hamilton – Another Wise Guy: • The famous mathematician Hamilton found that, in classical mechanics, energy calculations were often simplified if some familiar equations were recast in terms of momentum. In this way, momentum terms served to specify kinetic energy (potential energy is described separately). Hamilton & “Classical” Kinetic Energy: Class Example – Angular Momentum: • In a number of cases we will need to consider angular momentum. Common examples are the treatment of “spinning” electrons (spin angular momentum) and the rotation of molecules (molecular framework angular momentum). Starting with the EKinetic for the rotational energy of a one dimensional rod, derive an expression for EKinetic which employs an angular momentum term.