Math Review PPT
advertisement

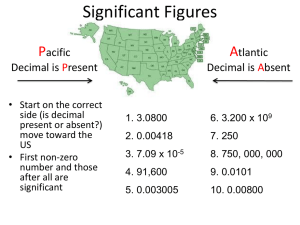
Problem Solving – a Math Review Unit 1 Significant Figures, Scientific Notation & Dimensional Analysis Significant Figures In science, we describe a value as having a certain number of significant figures or digits. – Includes all the #’s that are certain and 1 uncertain digit (the LAST one). – There are rules that dictate which #’s are considered significant! Rules for Significant Figures Any non-zero # is considered significant Zeroes! – Any zeroes between 2 numbers is significant Ex. 205 has 3 sig. figs. Ex. 4060033 has 7 sig. figs. Ex. 10.007 has 5 sig. figs. – Any zeroes before a number are NOT significant Ex. 0.054 has 2 sig. figs. Ex. 0.000 005 has 1 sig. fig. Rules for Significant Figures Zeroes! Continued – Any zeroes after numbers may or may not be significant. If there is a decimal point in the number, then YES, they are significant! – Ex. 12.000 has 5 sig. figs. – Ex. 0.1200 has 4 sig. figs. – Ex. 530.0000 has 7 sig. figs. If there is no decimal point in the number, then NO, they aren’t significant! – Ex. 120 has 2 sig. figs. – Ex. 430 000 000 000 has 2 sig. figs. Adding/ Subtracting and Significant Figures The rule – When adding or subtracting Look at the Significant Figures AFTER the decimal point. Which one has the least amount? That’s how many significant figures your answer can have Examples 17.34 + 4.900 + 23.1 = 45.34 (1 sig. fig after decimal) = 45.3 9.80 – 4.782 = 5.318 (2 sig. figs. After decimal) = 5.32 Multiplying/ Dividing and Significant Figures The rule – When multiplying or dividing, check out how many significant figures (all of them) each number has. Which one has the least amount? That’s how many significant figures your answer can have. Examples 3.9 × 6.05 × 420 = 9909.9 (2 sig. figs total) = 9900 = 9.9 × 103 14.2 ÷ 5 = 2.82 (1 sig. fig total) = 3 Scientific Notation Do you know this number? – 300 000 000 m/s – It’s the speed of light. Do you know this number? – 0.000 000 000 752kg – It’s the mass of a dust particle. Scientific Notation Instead of counting zeroes and getting confused, we use scientific notation to write really big or small numbers. – 3.00 × 108 m/s – 7.53 × 10-10 kg – The 1st number is the COEFFICIENT- it is always a number between 1 and 10. – The 2nd number is the BASE- it is the number 10 raised to a power, the power being the number of decimal places moved. Using a calculator with scientific notation A number written in scientific notation is NOT a math problem, it is a number in its own right. We put it into the calculator in a specific way! IF you have a scientific calculator, find the button that says EE or EXP. Scientific Calculators Scientific Calculators The EE or EXP button fills in for the × 10 part of the number written in scientific notation. Let’s say you are adding these two numbers 3.21 × 107 + 6.99 × 106 = This is how you would enter it into your calculator 3.21 EE 7 + 6.99 EE 6 = And you would get your answer. 3.91 × 107 Scientists generally work in metric units. Common prefixes used are the following: Dimensional Analysis is a problem-solving method that uses the fact that any number or expression can be multiplied by one without changing its value. It is a useful technique. The only danger is that you may end up thinking that chemistry is simply a math problem - which it definitely is not. Dimensional Analysis Unit factors may be made from any two terms that describe the same or equivalent "amounts" of what we are interested in. For example, we know that 1 inch = 2.54 centimeters We can make two unit factors from this information: Now, we can solve some problems. Set up each problem by writing down what you need to find with a question mark. Then set it equal to the information that you are given. The problem is solved by multiplying the given data and its units by the appropriate unit factors so that only the desired units are present at the end. (1) How many centimeters are in 6.00 inches? (2) Express 24.0 cm in inches. You can also string many unit factors together. (3) How many seconds are in 2.0 years? Density- What is it? Density is the ratio of mass to volume of a substance. – It can be used to identify a substance. – Ex. Water has a density of 1.00 g/mL – Ex. Gold has a density of 19.30 g/mL – Ex. Pumice has a density of 0.65 g/mL Density & Temperature Density = mass/ volume d = m/V Temperature = measure of the average kinetic energy a substance has – 3 scales Fahrenheit (°F) Celsius (°C) Kelvin (K) Temperature Scale Conversions From °C to °F T°F = 1.8(T°C) + 32° There are 3 temperature scales: From °F to °C T°C = .56(T°F - 32°) From °C to K T = T + 273 From K to °C T°C = TK - 273 – Fahrenheit (°F) – Celsius (°C) – Kelvin (K)