Concentration of solutions - Red Hook Central School District
advertisement
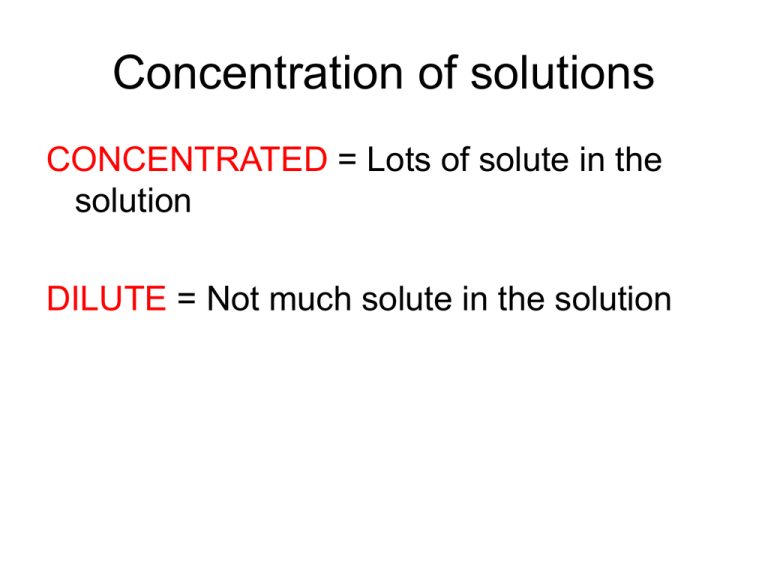
Concentration of solutions CONCENTRATED = Lots of solute in the solution DILUTE = Not much solute in the solution Ways to express concentration numerically • • • • Molarity % by Mass % by Volume Parts per million MOLARITY Molarity = # of moles of solute in 1 liter of solution See Table T Molarity Problems What is the molarity of a solution of NaOH if there are 4 moles of NaOH dissolved water to make 1 liter of solution? 4 moles 1 liter = 4M Molarity Problems What is the molarity of a solution of NaOH if there are 4 moles of NaOH dissolved water to make 2 liters of solution? 4 moles 2 liter = 2M Molarity Problems What is the molarity of a solution of NaOH if there are 2 moles of NaOH dissolved water to make 6 liters of solution? 2 moles 6 liters = .33 M Molarity Problems What is the molarity of a solution of NaOH if there are 2 moles of NaOH dissolved water to make .5 liters of solution? 2 moles .5 liters = 4M Molarity Problems What is the molarity of a solution of NaOH if there are 40 grams of NaOH dissolved water to make 1 liters of solution? First, convert grams to moles: Na = 23 g/mol O = 16 g/mol H= 1 g/mol 40 g/mole GFM so 40 g = 1 mole Molarity Problems What is the molarity of a solution of NaOH if there are 40 grams of NaOH dissolved water to make 1 liters of solution? 1 moles 1 liters = 1M Molarity Problems What is the molarity of a solution of NaOH if there are 80 grams of NaOH dissolved water to make 1 liters of solution? 80g/40g/mol = 2 moles 2 moles 1 liters = 2M Molarity Problems What is the molarity of a solution of NaOH if there are 3.6 grams of NaOH dissolved water to make 2 liters of solution? 3.6g/40g/mole = 0.09 moles 0.09 moles 2 liters = 0.045 M In General…. Concentrations < 1M are considered weak, or dilute Concentrations > 1M are considered strong, or concentrated Always know the concentration of your solution before you use it!!!! (read the label) Can you…. Rearrange the molarity equation to solve for moles? Molarity = Moles Liter moles = ? Can you…. Rearrange the molarity equation to solve for moles? Molarity = Moles Liter moles = Liter x Molarity Can you…. Rearrange the molarity equation to solve for Liters? Molarity = Moles Liter Liters = ? Can you…. Rearrange the molarity equation to solve for Liters? Molarity = Moles Liter Liters = Moles Molarity Example How many moles of NaOH do you have if you have 3 liters of a 0.5M NaOH solution? Moles = Molarity x Liters Moles = 0.5M x 3 liters Moles = 1.5 % Mass Uses the same formula as % composition (See Table T) Percent mass = mass of part Mass of whole x 100 % Mass What is the % mass of NaOH if 2.5 g of NaOH are added to 50 g of water? Percent mass = mass of part (solute) x 100 Mass of whole (solution) % mass = 2.5g (2.5 + 50g) x % mass = (2.5 / 52.5) x 100 = 4.76% solution 100 % Volume Uses the same formula as % composition (See Table T) Percent volume = volume of part volume of whole x 100 % Volume What is the % volume of NaOH if 20 ml of NaOH are added to 300 ml of water? Percent volume = volume of part (solute) volume of whole (solution) % volume = 20 ml (20 ml + 300 ml) % volume = (20 / 320) x 100 = 6.25% solution x 100 x 100 Parts per million ppm = grams solute x 1,000,000 grams solution This is used when the amount of solute is very very small Parts per million ppm = grams solute x 1,000,000 grams solution .0043 g of oxygen gas dissolve in 100 ml of water at 20 degrees celsius. What is the concentration in ppm? Parts per million ppm = .0043g x 1,000,000 (100 g + 0.0043g) Concentration = 43 ppm







