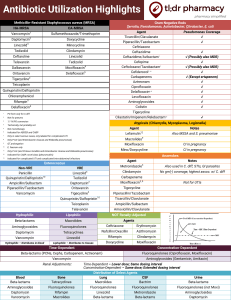
Susceptibility testing new agents eg. linezolid, tigecycline

Susceptibility testing new agents
.
Dr. Ian Morrissey
Chief Executive
GR Micro Ltd.
London, UK.
New Agents
• Linezolid (Pfizer)
• Tigecycline (Wyeth)
• Daptomycin (Novartis)
• Dalbavancin (Pfizer)
Basic principles still important.
Linezolid
• Approved in 2000 in the USA.
• Community-acquired and nosocomial pneumonia.
• Complicated and uncomplicated skin and soft-tissue infections.
• Methicillin-resistant Staphylococcus aureus resistant enterococci. and vancomycin-
• Protein synthesis inhibitor
• Bacteriostatic agent
• Oral and parenteral formulations.
Linezolid susceptibility
• Non-susceptibility is rare
– Some development signs in Enterococci esp. E. faecium .
– Occasional cases in S. aureus .
– Very rare in S. pneumoniae .
Linezolid MIC Breakpoints mg/L (≤S/>R)
Staphylococcus
Enterococcus
4/4
4/4
Streptococcus
A,B,C,G
S.pneumoniae
4/4
2/4
Non-species related 2/4
Zone diameters, 10 µg disc (mm)
≥S I ≤R
19
19
-
-
20
20
19
19
-
-
-
-
20
20
-
CLSI modified guidelines
• QC ranges changed from 27-31 mm to 25-32 mm after 4-5 yrs of use.
• Bacteriostatic agents may be more problematical when measuring zones or MICs by Etest.
Biedenbach & Jones 2003 CMI 9:1035
Tigecycline
• Launched 2005 in USA for SSTI.
• First glycylcycline
– Derivative of minocycline
– Evades Tet A-E & K efflux pumps
– But not those of Proteae or Pseudomonas
• Unique amongst newer agents having activity against Gramnegative bacteria.
– But is active against MRSA and VRE.
– Acinetobacter baumannii
• Parenteral administration only.
• Bacteriostatic.
Tigecycline susceptibility
• High against Gram-positive bacteria, E. coli & K. oxytoca .
• Some resistance found in Gram-negs
–
–
K. pneumoniae (92-95% susceptibility)
E. aerogenes (96% susceptibility)
–
–
E. cloacae (93% susceptibility)
S. marcescens (97% susceptibility)
Waites et al 2006 AAC 50: 3479
Tigecycline breakpoints
Tigecycline MIC Breakpoints mg/L (≤S/>R)
Enterobacteriaceae
Staphylococcus
Enterococcus
Streptococcus
Non-species related
1/2
0.5/0.5
0.25/0.5
0.25/0.5
0.25/0.5
Zone diameters, 15 µg disc (mm)
≥S I ≤R
19 20-23 24
25
20
19
-
-
-
-
-
26
21
20
-
Medium batch & Tigecycline activity
• Inconsistencies were noticed in QC data.
– Linked to medium batch variation.
• Tetracyclines are prone to inactivation by oxidation.
• Studies carried out to investigate.
–
–
Petersen & Bradford 2005 AAC 49:3910
Bradford et al 2005 AAC 49:3903.
Old vs new
Resolved by addition of Oxyrase
Chromatographic evidence
H
2
O
H
2
O & Oxyrase
Aged MHB
Medium effect
• Important for agar dilution or broth dilution.
– Media >12h old affected.
• Does not effect disc diffusion or Etest methods.
• If using broth microdilution frozen panels are also not affected.
• Solved by boiling or addition of oxyrase.
Daptomycin
• First glycolipopeptide
• Launched in 2003 in the USA – 2006 in Europe
– SSTI
– Endocarditis in the USA
• Gram-positives only
• Parenteral application only
• Rapidly bactericidal
Daptomycin Susceptibility
• Non-susceptibility rare.
– Some reports but case studies only.
Daptomycin MIC Breakpoints mg/L (≤S/>R)
Staphylococcus
[Enterococcus
Streptococcus
1/1
4/4]
1/1
[CLSI only]
≥S
-
Zone diameters (mm)
I
-
≤R
-
-
-
-
-
-
Daptomycin – requires Calcium
Calcium effect
MIC at zero Ca++ =
64 mg/L
GR Micro Ltd, data on file.
Daptomycin diffusion assays
• Daptomycin discs supplemented with 50 mg/L Ca++ have been developed.
– Only for CLSI methodology (i.e. MHB)
– Discontinued in 2005
• Isosensitest discs have proved problemmatical and have never been available.
Why are dapto & Ca++ discs no longer available even for CLSI?
Daptomycin treatment failure
Susceptible BP was ≥16 mm
Hayden et al 2005 JCM 43:5285.
Multicentre evaluation
Jevitt et al 2006 JCM 44:3098
Etest to the rescue!
Jevitt et al 2006 JCM 44:3098
Dalbavancin
• New lipoglycopeptide
– Derived from teicoplanin
• Extended half-life
– Once weekly dosing
• Bactericidal
• 8-16-fold more active than vancomycin
• No resistance found to date
– Except for cross-resistance to vanA Enterococci.
• Not clinically available but has ‘FDA Approval’
Dalbavancin Susceptibility
• Breakpoints not currently set.
• Broth microdilution requires addition of polysorbate-80 into wells for accurate and reproducible results.
• Agar diluton has not been proposed as a standard method
– Fritsche et al 2006 JCM 44:2988
• Problems with disc development
– Poor agar diffusion
– Jones et al 2006 JCM 44:2622
• Etest is available.
– Correct interpretation is essential.
Dalbavancin Etest
Biedenbach et al 2007 JCM 45:998
BSAC agar dilution method
• BSAC method compares well with CLSI (NCCLS)
– Mustaq et al 2004 JAC
54:617.
• Agar dilution commonly 1 dilution higher
Surrogate marker for dalbavancin
Jones et al 2006 JCM 44:2622
Summary
• Linezolid
– Continued use has ironed-out previous QC issues
• Tigecycline
– For broth: fresh medium or oxyrase is essential
• Daptomycin
– Discs not available – but Etests work.
– Ca++ supplement required
• Dalbavancin
– Some question over agar dilution but BSAC seems OK.
– Polysorbate-80 supplement in broth.
– No disc but Etest is available.