Understanding Table H and Vapor Pressure
advertisement

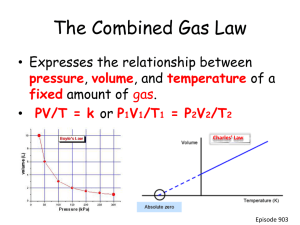
Understanding Table H and Vapor Pressure Take out your reference tables now In a closed system, at a steady temperature a dynamic equilibrium is reached. This is when the evaporation rate equals the condensation rate. This is only possible in a closed system. Corked top Air pressure outside bottle is about 101.3 Pa, same inside to start. Condensation (down arrows) WATER Evaporation (up arrows) If the system is heated, more evaporation occurs, until a new dynamic equilibrium is reached. If it’s cooled, less evaporation will occur, until a new dynamic equilibrium is reached. Corked top Air pressure outside still about 101.3 kPa, inside the pressure is increasing. WATER Condensation (down arrows) Evaporation (up arrows) The extra pressure inside that bottle is called the vapor pressure. It’s added to the existing air pressure present from the start. Corked top Air pressure outside still about 101.3 kPa, inside the pressure is increasing. WATER Condensation (down arrows) Evaporation (up arrows) The vapor pressure of water (part of table H) 101.3 kPa Pressure kPa 0 50 100 temperature, Centigrade degrees The vapor pressure of water (part of table H) Big black dot indicates the “normal boiling point”, the BP at normal pressure. 101.3 kPa The curved line indicates ALL of the boiling points of water, at all different pressures. Pressure kPa 0 50 100 temperature, Centigrade degrees The vapor pressure of water (part of table H) H2O at these pressures and temperatures is a 101.3 kPa LIQUID H2O at these pressures and temperatures is a Pressure kPa GAS 0 50 100 temperature, Centigrade degrees Table H is the vapor pressure of 4 different compounds, water included. Only look at one curve, or one liquid, at any time. Behind the curve is liquid. In front is a gas. Table H is the vapor pressure of 4 different compounds, water included. Only look at one curve, or one liquid, at any time. Behind the curve is liquid. In front is a gas. Let’s talk ONLY about water… At each point, what phase is the water in? 1. 2 1 4 2. 3. 3 4. Table H is the vapor pressure of 4 different compounds, water included. Only look at one curve, or one liquid, at any time. Behind the curve is liquid. In front is a gas. Let’s talk ONLY about water… At each point, what phase is the water in? 1. liquid 2 1 4 2. liquid 3. liquid 3 4. gas At each point, what is the vapor pressure of water? 101.3 kPa + 40⁰C 75 kPa + 95⁰C 150 kPa + 110⁰C 20 kPa + 65⁰C At each point, what is the vapor pressure of water? 101.3 kPa + 40⁰C LIQUID 75 kPa + 95⁰C GAS 150 kPa + 110⁰C LIQUID 20 kPa + 65⁰C GAS 13 At points A, B, and C What phase is water? What phase is ethanoic acid? A C B What phase is ethanol? What phase is propanone? At points A, B, and C What phase is water? A liquid B gas C liquid A C B At points A, B, and C What phase is ethanoic acid? A C B A liquid B liquid C liquid At points A, B, and C What phase is ethanol? A C B A liquid B gas C gas At points A, B, and C What phase is propanone? A C B A gas B gas C gas How much extra pressure is added to a sealed flask containing water at 50⁰C? (what’s the vapor pressure of water at 50⁰C?)