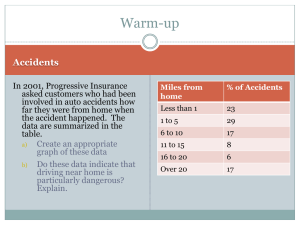
Accompanying Presentation
advertisement

Introduction to Møller-Plesset Perturbation Theory Kelsie Betsch Chem 381 Spring 2004 Møller-Plesset: Subset of Perturbation Theory Rayleigh-Schrödinger Perturbation Theory H = H<0> + V Møller-Plesset Assumption that H<0> is Hartree-Fock hamiltonian Parts of the hamiltonian H<0> is Hartree-Fock operator Counts electron-electron repulsion twice V corrects using Coulomb and exchange integrals gij = fluctuation potential H 0 N N N N Fi ( h i J ij K ij ) ) h i 2 V ee i 1 V HH i 1 0 j 1 (J i , j 1 h N i i 1 i 1 N V ee N N ij K ij ) V ee 2 V ee i 1, j i g ij i, j N g ij g i , j 1 ij Complete Hamiltonian and Energy Expression Complete Hamiltonian Hartree-Fock energy is sum of zeroth- and firstorder corrections Expression for correlation energy N H h N i i 1 g ij i 1, j i EHF = E0<0> + E0<1> E corr n2 n E0 Calculating Correlation Energies Promote electrons from occupied to unoccupied (virtual) orbitals MP with 2nd order correction (MP2) Electrons have more room Decreased interelectronic repulsion lowers energy Two-electron operator Single, triple, quadruple excitations contribute nothing Corrections to other orders may have S,D,T,Q, etc. contributions Select methods may leave some contributions out (MP4(SDQ)) How close do the methods come? MP2 ~ 80-90% of correlation energy MP3 ~ 90-95% MP4 ~ 95-98% Higher order corrections are not generally employed Time demands How to make an MP calculation Select basis set Carry out Self Consistent Field (SCF) calculation on basis set Obtain wavefunction, Hartree-Fock energy, and virtual orbitals Calculate correlation energy to desired degree Integrate spin-orbital integrals in terms of integrals over basis functions Basis Set Selection Ideally, complete basis set Complete basis sets not available Yields an infinite number of virtual orbitals More accurate correlation energy Finite basis sets lead to finite number of virtual orbitals Less accurate correlation energy Smallest basis set used: 6-31G* Error due to truncation of basis set is always greater than that due to truncation of MP perturbation energy (MP2 vs. MP3) Advantages and Disadvantages PT calculations not variational Difficult to make comparisons No such upper bound to exact energy in PT as in variational calculations PT often overestimates correlation energies Energies lower than experimental values Advantages and Disadvantages Interest in relative energies Variational calculations, such as CI, are poor MP perturbation theory is size-extensive Gives MPPT superiority MP calculations much faster than CI Most ab initio programs can do them MP calculations good close to equilibrium geometry, poor if far from equilibrium Summary Møller-Plesset perturbation theory assumes Hartree-Fock hamiltonian as the zero-order perturbation Hartree-Fock energy is sum of zerothand first-order energies Correlation energy begins with secondorder perturbation How an MP calculation is carried out Strengths and weaknesses of MP vs. CI Acknowledgements Dr. Brian Moore Dr. Arlen Viste References P. Atkins and J. de Paula, Physical Chemistry, 7th ed. W.H. Freeman and Company, New York, 2002. A. Szabo and N.S. Ostlund, Modern Quantum Chemistry: Introduction to Advanced Electronic Structure Theory, Dover Publications, Inc., Mineola, NY, 1989. C. Møller and M.S. Plesset, Phys. Rev., 46:618 (1934). F.L. Pilar, Elementary Quantum Chemistry, 2nd ed. Dover Publications, Inc., Mineola, NY, 1990. F. Jensen, Introduction to Computational Chemistry, John Wiley & Sons, Chichester, 1999. E. Lewars, Introduction to the Teory and Applications of Molecular and Quantum Mechanics, Kluwer Academic Publishers, Boston, 2003. I.N. Levine, Quantum Chemistry, 5th ed. Prentice Hall, Upper Saddle River, NJ, 2002 .