Lect4
advertisement

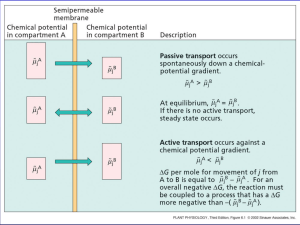
• Announcements Today Review membrane potential What establishes the ion distributions? What confers selective permeability? Ionic basis of membrane potential Next Lectures Action Potentials • Membrane Potential – Inside of cell is negative compared to outside – Depends on: – High concentration K+ inside – Selective permeability of membrane What causes the different ion distributions in cells? 1. Passive distribution – Donnan equilibrium 2. Active Transport Passive distribution – Donnan equilibrium • The ratio of positively charged permeable ions equals the ratio of negatively charged permeable ions Start I Equilibrium II K+ Cl- I II [K+] = [K+] [Cl-] = [Cl-] Donnan Equilibrium • Mathematically expressed: [ K ]I [ K ] II [ C l ] II [C l ]I •Another way of saying the number of positive charges must equal the number of negative charges on each side of the membrane Passive Distribution • BUT, in real cells there are a large number of negatively charged, impermeable molecules (proteins, nucleic acids, other ions) • call them AStart I AK+ Cl- Equilibrium II I A[K+] > [K+] [Cl-] < [Cl-] II Passive Distribution Equilibrium I A- II [K+] > [Cl-] < [Cl-] +’ve = -’ve [K+] [K+]I = [A-]I + [Cl-]I [K+]II = [Cl-]II If [A-]I is large, [K+]I must also be large +’ve = -’ve space-charge neutrality • The presence of impermeable negatively charged molecules requires more positively charged molecules inside the cell. Donnan Equilibrium Example Initial Concentrations I II A- = 100 A- = 0 K+ = 150 K+ = 150 Cl- = 50 Cl- = 150 Are these ions in electrochemical equilibrium? No, EK+ = 0 mV ECl- = -27 mV [ K ]I [ C l ] II [ K ] II [C l ]I 150 X 150 X 150 X Let X be the amount of K+ and Cl- that moves 50 X Solve for X, 7500 + 200X + X2 = 22500 - 300X + X2 X=30 Final Concentrations I II A- = 100 A- = 0 K+ = 180 K+ = 120 Cl- = 80 Cl- = 120 space-charge neutrality Are these ions in electrochemical equilibrium? Yes, EK+ = -10 mV ECl- = -10 mV What causes the different ion distributions in cells? 1. Passive distribution – Donnan equilibrium 2. Active Transport Active Transport • ATP-powered pumps – Proteins that are capable of pumping ions from one side of the cell membrane to the other – Use energy Active Transport • Na+ - K+ pump 2 K+ outside inside 3 Na+ ATP ADP + Pi Active Transport • Na+ - K+ pump – 3 Na+ move out – 2 K+ move in – Hydrolyzes ATP • Maintains the concentration gradient Active Transport • Na+ - K+ pump 2 K+ outside inside 3 Na+ Electrogenic • net loss of 1 positive charge from inside • Inside becomes more negative • contributes a few mV to resting potential • Na+/K+ pump is required – Due to low permeability for Na+ to leak into the cell – Without pump, Gradual accumulation of +’ve charge inside Eventually lose the membrane potential Active ingredient in rodent poison, ouabain, poisons the Na/K pump What causes the different ion distributions in cells? 1. Passive distribution – Donnan equilibrium 2. Active Transport What confers selective permeability? Ion channels – Non-gated – Leakage channels – Open at rest – allow K+ to flow out along its concentration gradient K+ Membrane Potential Summary 1. Selective permeability • Ion channel – K+ leak channel 2. Unequal distribution of ions • • Passive distribution (Donnan) Active transport – Na+/K+ pump 3. The equilibrium potential of each ion is described by the Nernst equation 4. The total membrane potential is described by the Goldman equation • Ionic basis of membrane potential Mammalian Cell Inside (mM) Outside (mM) Equilibrium (Nernst) Potential K+ 140 5 -84 mV Na+ 10 120 +63 mV Cl- 4 110 -83 mV Ca2+ 0.001 5 +107 mV Is the resting membrane potential controlled by one ion, or several? • Do an experiment – Measure membrane potential (Vm) of a cell – Change extracellular concentration of an ion in the bathing solution – If Vm really depends on Eion than Vm should change as Eion changes Measuring Membrane Potential amplifier microelectrode Reference electrode Resting potential 0 mV cell -80 mV time Expt #1 vary extracellular Na [Na]out Assume [Na]in = 10 mM 1 5 10 20 100 200 prediction E N a 0.058 log [ N a ]out [ N a ]in -58 mV -17 0 17 58 75 40 Membrane Potential (mV) 20 0 -20 Prediction of ENa From Nernst equation -40 -60 -80 -100 -120 Measured Vm 1 2 5 10 20 50 100 200 External Na+ concentration (mM) • Therefore, – Conclude that Vm does not follow ENa Expt #2 vary extracellular K Extracell [K] • Assume intracellular [K] = 140 mM 1 5 10 20 100 200 prediction E K 0.058 log [ K ]out [ K ]in -124mV -84 -66 -49 -8 9 Membrane Potential (mV) Deviation at low [K+] due to slight permeability of Na+ 0 -20 Prediction of EK From Nernst equation -40 -60 -80 -100 -120 Measured Vm 1 2 5 10 20 50 100 200 External K+ concentration (mM) • Therefore, the resting membrane potential (Vm) is very close to the equilibrium potential for K+ (EK) Summary 1. At rest PK>>PNa, PCl, PCa 2. Therefore, at rest, the membrane potential is close to EK 3. In general, the membrane potential will be dominated by the equilibrium (Nernst) potential of the most permeable ion Sample Question At rest Vm of this typical cell is -75 mV. What would Vm be if PNa >> Pk,PCl? K = 140 Na = 10 Cl = 30 K=5 Na = 145 Cl = 110 Answer: Calculate ENa using Nernst equation. Assume Vm ENa = +67 mV • The resting membrane potential is the basis for all electrical signaling • Next Lecture: Action Potentials