Molar Quantities Notes

Chemical Quantities
Subatomic Particles
Subatomic particles include the proton, neutron, and electron
Which of the following does not exist in the nucleus?
1.
2.
3.
Proton
Neutron
Electron
P ro to n
0% 0%
N eu tro n
0%
El ec tro n
Which of the following has a mass much less than the other two particles?
1.
2.
3.
Proton
Neutron
Electron
P ro to n
0% 0%
N eu tro n
0%
El ec tro n
Which of the following has an electric charge of zero?
1.
2.
3.
Proton
Neutron
Electron
P ro to n
0% 0%
N eu tro n
0%
El ec tro n
Key terms of atomic structure
Atomic Number (Z) = number of protons
Each element has its own unique atomic number
Mass number = sum of protons and neutrons
= mass of the nucleus
Isotope Notation
Isotopes are forms of the same element
(same number of protons) with differing numbers of neutrons
How many protons are in the following isotope?
1.
2.
3.
19
9
10
19
0% 0%
9
0%
10
How many neutrons are in the following isotope?
1.
2.
3.
19
9
10
19
0% 0%
9
0%
10
Atomic Mass
The weighted average of all natural occurring isotopes of that element
Example: Lithium = 6.941 amu
Atomic number closest to 7, therefore most isotopes of lithium have a mass number of 7
Rubidium has two common isotopes, 85Rb and 87Rb. If the abundance of
85Rb is 72.2% and the abundance of
87Rb is 27.8%, what is the average atomic mass of rubidium?
.722 x 85
(%) times (mass number)
+
.278 x 87
(%) times (mass number)
=
85.55
Chemical Quantities
Chemical formulas give essential information about the exact ratio of atoms that form a substance.
Ex. CO
2
- carbon dioxide contains a 1:2 ratio of carbon to oxygen atoms.
The subscript (smaller number) represents the number of atoms in a molecule.
How many oxygen atoms are in one molecule of NaC
2
H
3
O
2
?
1.
2.
3.
4.
5.
6.
3
4
1
2
6
12
1
0%
2
0%
3
0% 0%
4
0%
6
0%
12
How many nonmetal atoms are in one molecule of NaC
2
H
3
O
2
?
1.
2.
3.
4.
5.
6.
3
5
1
2
7
8
1
0%
2
0%
3
0% 0%
5
0%
7
0%
8
How many oxygen atoms are in one molecule of Co(NO
3
)
2
?
1.
2.
3.
4.
5.
6.
3
4
1
2
6
12
1
0%
2
0%
3
0% 0%
4
0%
6
0%
12
How many nitrogen atoms are in one molecule of Co(NO
3
)
2
?
1.
2.
3.
4.
5.
6.
3
4
1
2
6
12
1
0%
2
0%
3
0% 0%
4
0%
6
0%
12
How many oxygen atoms are in one molecule of Fe
3
(PO
4
)
2
?
1.
2.
3.
4.
5.
6.
7.
3
4
1
2
6
8
12
0% 0% 0% 0% 0% 0% 0%
1 2 3 4 6 8
12
Chemical Formulas
If there are more than one molecule, a
coefficient is used to show how many molecules are present.
3H
2
O represents 3 water molecules.
3 is the coefficient.
2 is the subscript for hydrogen, showing there are two hydrogen atoms in each molecule
How many sodium atoms are in
3NaC
2
H
3
O
2
?
1.
2.
3.
4.
5.
6.
3
5
1
2
7
8
1
0%
2
0%
3
0% 0%
5
0%
7
0%
8
How many hydrogen atoms are in
3NaC
2
H
3
O
2
?
1.
2.
3.
4.
5.
6.
7.
3
6
1
2
7
8
9
0% 0% 0% 0% 0% 0% 0%
1 2 3 6 7 8 9
How many nitrogen atoms are
2Co(NO
3
)
2
?
1.
2.
3.
4.
5.
6.
3
4
1
2
6
12
1
0%
2
0%
3
0% 0%
4
0%
6
0%
12
How many oxygen atoms are
2Co(NO
3
)
2
?
1.
2.
3.
4.
5.
6.
3
4
1
2
6
12
1
0%
2
0%
3
0% 0%
4
0%
6
0%
12
How thick is a page of your textbook?
Here … take a ruler and start measuring.
Okay, now what about a container of paperclips, how can we tell “how many” without counting all of them?
Now how many are in this other package of paperclips – same size package?
Why are the amounts of paper clips different even though the size of the package is the same?
They’re Different Substances!!
A little review …
What is average atomic mass?
Atomic mass is based on the weighted average of all of an atom’s isotopes.
So one nitrogen atom has a mass of 14.01 a.m.u. (atomic mass units)
Now apply this to compounds …
For molecules compounds, we must add the individual atomic masses of each atom.
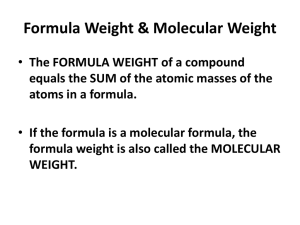
Molecular mass – sum of the atomic masses in a molecular (covalent) compound.
Formula mass – sum of all the masses in the formula of an ionic compound
Let’s try to find:
- the molecular mass of C
2
H
5
OH
- the formula mass of (NH
4
)
3
SO
4
But we can’t count individual atoms easily, so what should we use instead?
The Mole (not the blind rat thing…) vs.
Formula and Molecular Mass
Mole – SI unit for amount
-think of a mole like you think of a dozen eggs.
One mole has 6.02*10 23 atoms/molecules/formula units/ions/ leprechauns/ cowbells/ Bengal fans
Moles in a Conversion Factor
1 mole = 6.02 x 10 23 of anything
Conversion factor (for moles to atoms):
1 mole atoms = 6.02 x 10 23 atoms
6.02 x 10 23 atoms 1 mole atoms
Write the given information 1 st and then choose which conversion factor to use.
Rarely, though, do we deal in terms of individual atoms or molecules.
Moles deal in grams
- How can we go from moles to grams?
Molar Mass
Molar mass – has same value as molecular mass or formula mass, but instead of measuring at atomic level, measures at the molar level
- units in grams per mole, not amu
- calculated same way, uses atomic masses
Let’s try to calculate the molar mass of
C
6
H
6
.
Now let’s convert 11.5 g of ethanol, C into moles.
2
H
5
OH
Okay, good, now let’s convert 1.20*10 25 molecules of NH3 to moles, then to grams.
What is the mass in a.m.u. of 100 atoms of nitrogen?
How many atoms of potassium are there if a sample has a mass of 7.82 x 10 10 a.m.u.?
Percentage Composition
How can we find what percentage by mass of the Cleveland Browns starting offense is from the offensive linemen?
1 quarterback – 220 lb / each
2 running backs -
2 wide receivers –
200 lb / each
190 lb / each
1 tight end 235 lb
5 offensive linemen 340 lb / each
1 coach 400 lb
Let’s try to find out what percentage by mass of water is made up of hydrogen and what percentage is oxygen…
Percentage composition – statement of the relative mass each element contributes to the mass of the compound as a whole.
Steps:
1. find total molar mass of compound
2. find the total mass of the element present in the compound
3. put the mass of the element over the mass of the compound, divide and multiply by 100%.
Find the percent mass of carbon in C
2
H
5
OH.
Empirical and Molecular Formulas
Empirical Formula – the simplest whole number ratio of atoms in a compound
(ex. C
6
H
12
O
6 becomes C H
2
O)
Molecular Formula – the actual whole number ratio of atoms in a compound
(sometimes it is the same as the empirical formula) (ex. C
6
H
12
O
6
)
Determining Empirical Formulas
When we form a compound, the atoms combine in whole number ratios
- if we have information on the masses of certain elements present, we can determine empirical formulas
Steps to Calculate the Empirical Formula:
1. Turn everything into moles – do not round.
2. Change into simple ratios by dividing all amounts by the value with the lowest number of moles.
3. If the ratio is not close to a whole number, multiply by a number which will make it so
- for example, if you have a 1.47, this is close to neither 1 nor 2, so we would need to double
1.47 and any other values.
4. Round off when you are finally close to a whole number.
Example: Say we have a compound w/
.900 g of Ca and 1.60 g of Cl. Find the empirical formula.
It is important to remember that if you are given percent composition, it is referring to mass.
- treat as if they were 100 g samples
- 12.3 % Cl becomes 12.3 g of Cl
Ex: What is the empirical formula of a compound that is 66.0% Ca and 34.0% P?
Determining Molecular Formula
In order to determine a molecular formula, we need a couple of things:
- molar mass
- empirical formula
Divide the molar mass of the molecular formula by the molar mass of the empirical formula to get a whole
number.
- multiply through empirical formula by this whole number
Ex: Find the molecular formula of a substance w/ an empirical formula of
AgCO
2 and a molar mass, of the true molecular formula, of 304 g.
Find the molecular Formula
Testosterone has a molecular weight of
282.42 g.
C 79.12%, H 9.79%, O 11.09%
Hydrates
Some compounds, when formed actually contain water as part of their formula
- not a solution
- think of a button in a pocket, and a button sewn onto a pocket
- crystallize out of water and just happen to take some of the water molecules with them
Hydrates – ionic compounds or molecules chemically bonded to water
(ex: CuSO
4
.
5H
2
0) copper (II) sulfate pentahydrate
This tells us that there are five water molecules bonded to every one of the copper (II) sulfates
Much like in finding an empirical formula, we can find the formula of a hydrate given information about the masses of water and compound.
Steps:
1. find the mass of the compound and the mass of the H
2
O
2. change both to moles
3. divide both by lowest number of moles
4. use results as coefficients
Special note, if a hydrate is heated, the difference between the mass before and the mass after is equal to the mass of the water.