2011 Data Analysis
advertisement
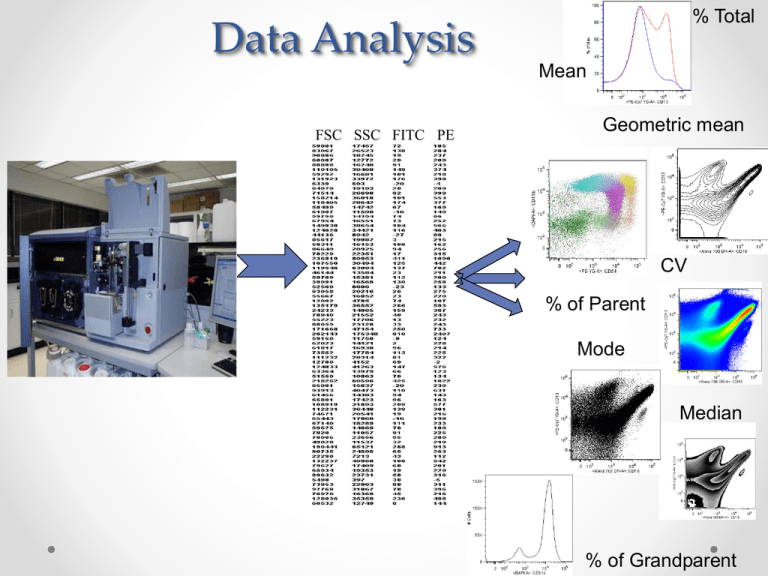
Data Analysis FSC SSC FITC PE % Total Mean Geometric mean CV % of Parent Mode Median % of Grandparent Data Analysis Experimental Design Sample Procurement Sample preparation Fix/Perm Which Fluorophore Controls Isotype? Single color FMO Instrumentation Appropriate Lasers Appropriate Filters Instrument Settings Lin vs Log Time A, W, H Interpretation Mean, Median %+ CV SD Signal/Noise Gating Analysis Presentation Histogram Dot Plot Density Plot Overlay Bar Graph Objective This will involve both the “what is” data analysis and the “how to”. • Instrumentation (Mostly covered in “Flow Basics”) • Underlying Principles/Concepts • Proper Technique First, lets address the problems • Data analysis incorporates many disciplines including instrumentation, statistics, biology, and photonics. Often times knowledge in one of these is limited • Many different instruments and software packages are available. • Historical precedento Unfortunately there is a large body of work published with poor data and no clear guidelines Flow Basics • A quick look back to Flow Basics to see what we are analyzing Photons ElectronsVoltage pulseDigital # Measurements of the Pulse Pulse Width Voltage Intensity Pulse Height Pulse Area Time 1 256 10,000 196 1000 .1 (Volts) 3.54 volts (Volts) 6.21 volts 128 .01 64 .001 (1mV) 0 1.23 volts 0 Photons=voltage=relative brightness 100 10 1 Relative Brightness 10 Channel Number 10 FCS File or List Mode File FCS File- The flow cytometry data file standard provides the information needed to completely describe flow cytometry data sets within the confines of the file containing the experimental data. It is made up of the Header, Text and Data. Current version is FSC 3.0 Data FSC SSC FITC PE APC APC-Cy7 FCS file Text Keywords- value pairs that describe the experiment, the instrument, the specimen, the data and any other information which the file creator wishes to include. Some values are user defined, others are automatically entered Histograms 120 N=552 Frequency 100 80 60 40 20 0 1 3 5 7 9 11 13 15 17 19 # of Apples picked per tree Frequency N=7696 0 101 102 103 104 Arbitrary Fluorescent units Histogram- is a summary graph showing a count of the data points falling in various ranges. The effect is a rough approximation of the frequency distribution of the data. Creation of a Histogram Listmode File Event # 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 FSC 500 645 6416 64186 313 354684 654165465 51 5165 5418 654164 3135 684 6541 3546 35416 3154564 1354 3154 164 SSC PE 6176 6146 3543 315 34 3054 3054 8668 6840 80 9354 35 48979 540 354 354 304 354 68 68 9 2013 3455 251 11 1265 25 659 6145 256 3698 4848 5 100 5468 154 8 7 9852 3652 12 9 6 3 0 101 102 PE 103 104 Dot Plots Dot plot- Frequency distributions of two simultaneously displayed parameters Plots Contour Pseudo-color Histogram Greyscale Density Dot plot Dot plots vs density or Pseudocolor 6,000,000 Events 1,500,000 6,000,000 4,500,000 600,000 300,000 3,000,000 150,000 Gating Selecting a subset of cells based on a specific fluorescent or scatter pattern. -Either graph can be OK -However “gated” may be more appropriate due to exclusion of artifact and non T cells No Gate CD3+ Gate 11.3% 59.8% 5.8% 30% Interpretation 11.3% of the WBC’s are positive for CD4 and 5.8% are positive for CD8 Interpretation 59.8% of the CD3+ cells are positive for CD4 and 30% are positive for CD8 Hierarchical Gating Compensation Fluorescent Compensation- Due to broad emission profiles of fluorescent dyes, a single detector may process light from multiple dyes. The process of mathematically correcting for this spectral overlap is called compensation Compensation Mean/Median/GeoMean • Mean • Sum of the “n” individual values of a group divided by n • NOT GOOD FOR Log DATA • More easily skewed by outliers • Geometric Mean • Multiply the ‘N” values of a cluster together and get the nth root of this product • Better for log data • Median • Divides your data in half • Probably the safest for Log data http://flowjo.typepad.com/the_daily_dongle/2007/10/mean-median-mod.html Location of mean, median and mode with different distributions. Normal Left skew Right skew Driscoll P et al. J Accid Emerg Med 2000;17:274-281 Objective This will involve both the “what is” data analysis and the “how to”. • Instrumentation (Mostly covered in “Flow Basics”) • Underlying Principles/Concepts • Proper Technique There is no wrong way to analyze your data Meaning- Investigators are free to choose: • Which plot types for display • Placement of gates for analysis • Which statistics • # events to display or collect • Which software package to use • How many times you reanalyze There is definitely a wrong way to analyze your data Meaning- Investigators decisions can lead to incorrect data generation or interpretation: • Inappropriate gates for analysis (lymphocyte gate for CD15 staining, or inconsistent gates) • Misleading or inconsistent plots for display • Inappropriate controls (e.g. using isotype for gating) • Inappropriate number of events collected (too few events for meaningful and accurate statistical comparison) Dot plot Vs Histogram Doublet discrimination Height • Cell cycle • Rare events • Dim cell lines Width Red=Singlets Blue=Doublets Fiona Craig Flow Cytometric Immunophenotyping of Cerebrospinal Fluid SpecimensAJCP 2011 135:22-34 Cell aggregates were identified in 29 (16.4%) of 177 CSF specimens and represented 0 to 1,503 events and 0% to 80% of total acquired events On axis data Bi-exponential Scaling • • • • Approximates log scaling at the upper end Approximates linear scaling at the lower end Can display events at or below zero Improves visual separation of population at the low end of the scale http://ucflow.blogspot.com/2011/04/displaytransformation-and-flowjo.html Log scale Bi-exponential Herzenberg et al Isotypes • Must be the exact same isotype • Conjugated to exactly the same degree Fluorophore/Protein ratio • Has the same background binding characteristics as your antibody • Different vendors use different conjugation protocols which may cause different characteristics • Used at the same concentration • Not used for gating purposes!!! Rarely is more than the first criterion met. FMO controls FMO = Fluorescence minus one The gating control contains all the fluorophores except the PE one of interest FITC Perfetto et al Rare events The essential feature of Poisson distributions is that if N events are observed the standard deviation (SD) associated with that count is square root of N . The coefficient of variation (CV) is then given by CV = 100 X SD/ N or 100 / sqrt N. How many events to detect is more of a statistics questions than a flow cytometry question Statistical Significance of Results • Determining the Number of Events to Collect Rare Events Case Study #1 • Number of Events vs Measurement Precision .1%-.18% .07%-.1% Rare Events Case Study #2 • Normal Kappa/Lambda ratio on B cells is .9-3.5 (polytypic) • When values fall outside this range it’s “clonal” • Often times in patients undergoing therapy there is a very low frequency of B cells to enumerate. Gated on CD19+ Bcells 20 Kappa/Lambda=58/20=2.9(Polytypic) But, √58=8 and √20=5 So actual values are 58±8/20±5 Which leads to ratio’s between 4.4 and 2. Is this clonal??????? 58 4 decade or 5 decade display FACSDiVa • • • The Diva system first converts the analog signal to digital using a 14-bit ADC (16, 384 bins), then when the data is log transformed, it is multiplied by 16 (or 24) effectively adding 4 bits to the data making it 18-bit (262,144 bins). It displays this data on a 5-decade scale, but then does not display the first decade. What you’re left with is a 4-decade log scale (actually the scale goes above the 4th decade a little bit) with bins from 262,144 down to 262. Decade Analog PMT Signal 14-bit Bins Upscaled to 18-bit Decade Range 5 10V 16384 262144 10001-100000+ 4 1V 1638 26214 1001-10000 3 .1V 164 2621 101-1000 2 .01V 16 262 11-100 1 .001V 2 26 1-10 Dead Cell Exclusion Immunophenotyping Despite light scatter gate Rare event analysis Prefetto et al JIM 2006 Dump gate With dump gate Without dump gate Light Scatter Laser Beam FSC Detector Collection Lens SSC Detector Original from Purdue University Cytometry Laboratories Why Look at FSC v. SSC • Since FSC ~ size and SSC ~ internal structure, a correlated measurement between them can allow for differentiation of cell types in a heterogenous cell population Granulocytes Dead SSC Lymphocytes LIVE Monocytes RBCs, Debris, Dead Cells FSC Kinetic and Ratio parameters Automated clustering algorithms Aghaeepour N, Nikolic R, Hoos HH, Brinkman RR. Rapid cell population identification in flow cytometry. Cytometry A. 79(1):6-13, 2011. Zare H, Shooshtari P, Gupta A, Brinkman RR. Data reduction for spectral clustering to analyze high throughput flow cytometry data. BMC Bioinformatics. 11(1): 403, 2010. Available Spectra Viewers • http://www.bdbiosciences.com/research/multicolor/spectrum_viewer/index.jsp • http://www.invitrogen.com/site/us/en/home/support/Research-Tools/FluorescenceSpectraViewer.html • http://www.biolegend.com/spectraanalyzer Data Analysis Feedback http://www.surveymonkey.com/s/K7WYTH7 References -Perfetto S, 2006 JIM 313 pg199-208 (live vs dead) -Keeney et al 1998 Cytometry -Cytometry 30(5), 1997 http://ucflow.blogspot.com/2011/04/display-transformation-and-flowjo.html (bi-exponential display) -Cytometry A 783A:384-385 -Seventeen-colour flow cytometry: unravelling the immune system -Stephen P. Perfetto, Pratip K. Chattopadhyay & Mario Roederer Nature Reviews Immunology 4, 648-655 (August 2004) -Interpreting flow cytometry data: a guide for the perplexed Herzenberg L, 2006 Nature Immunology Vol 7 Num 7 A practical approach to multicolor flow cyometry for immunophenotyping Baumgarth N, 2000 J. Imm. Methods 243 77-97 -Isotype controls in the Analysis of Lymphocyte and CD34+ stem and progenitor cells by Flow Cytometry-Time to Let Go! Keeney, M 1998 Cytomery (Communication in Clincal Cytometry 34: 280-283 # of Parameters 1 2 3 4 5 6 7 8 9 10 # of possible plots 1 1 3 6 10 15 21 28 36 45