Thermodynamics
advertisement

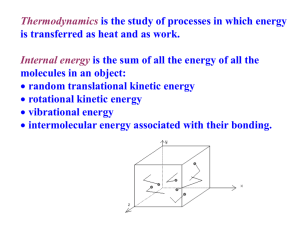
Thermodynamics -chap. 15 note for AP B physics: if it says ON gas, use W= -PDV if it says BY gas, use W= PDV always: U= Q + W The Ideal Gas Law (review chap.14) P V = N kB T N = number of molecules » N = number of moles (n) x NA molecules/mole kB = Boltzmann’s constant = 1.38 x 10-23 J/K PV=nRT R = ideal gas constant = NAkB = 8.31 J/mol/K NA = Avogrado’s number = 6.02 x 1023 Summary of Kinetic Theory: The relationship between energy and temperature (for monatomic ideal gas) ave KE/molecul e Root-mean-square speed : v rms v 2 1 m v 2 2 3k B T M 3 2 3RT k BT Careful with units: R = 8.31 J/mol/K = molar mass in kg/mol vrms = speed in m/s Internal Energy U = number of molecules x ave KE/molecule = N (3/2) kBT = (3/2) n RT = (3/2) P V (ideal gas) Thermodynamics a system (gas) interacts with surroundings via thermal processes (heat and work transferred into gas) : system P,V,T Example: surroundings gas piston (car engine, bike pump, syringe) system = gas at pressure P and temperature T and volume V surroundings = piston and walls of container state of system: values for P,V, and T describe gas The 4 Laws of Thermodynamics 0. Law: Two objects at thermal equilibrium will have no heat flow between them and the same temperature. T 1 = T2 1st law: Total internal energy = heat put in + work done on it U= Q + W 2nd law: Heat will spontaneously flow from hot to cold Carnot engine: ideal efficiency= (Hot- cold temp) /hot temp (otherwise: efficiency… use heat’s Q, not temp’s T) 3rd law: You can’t get to absolute zero! (T= OK) (can’t remove all the energy w/o still adding some) First Law of Thermodynamics “The internal energy of a system tend to increase when HEAT is added and work is done ON the system.” Suggests a CHANGE or subtraction D U Q W D U Q Add W on Q > 0 : heat is added to system Q < 0 : heat is subtracted from system W > 0 : work done on system by surroundings W < 0 : work done on surroundings by system Internal Energy (DU) and Heat Energy (Q) All of the energy inside a system is called INTERNAL ENERGY, DU. When you add HEAT(Q), you are adding energy and the internal energy INCREASES. Both are measured in joules. But when you add heat, there is usually an increase in temperature associated with the change. DU DT if D T , D U if D T 0 , D U 0 Thermodynamic Systems and P-V Diagrams Ideal gas law: P V = n R T For n fixed, P and V determine the “state” of the system T = P V/ (n R) U = (3/2) n RT = (3/2) P V Examples: P which point has highest T ? 1 2 P1 »2 which point has lowest U ? 3 P 3 »3 to change the system from 3 to 2, V1 V2 energy must be added to system. V Work done in a thermal process is the area underneath the P,V graph. Work done by a gas Suppose you had a piston filled with a specific amount of gas. As you add heat, the temperature rises and thus the volume of the gas expands. The gas then applies a force on the piston wall pushing it a specific displacement. Thus the gas does NEGATIVE work (uses its own internal energy to do work). Work is the AREA of a P vs. V graph W= -PDV = -P* (Vf- Vi) the work done on the gas Compress gas: gas expands itself smaller volume (DV=-) larger volume (DV=+) positive work more (W=+) negative work (W=-) gas has more energy gas loses energy move left on P-V diagram move right on P-V diagram -Work: clockwise +Work: counterclockwise Negative or positive Work Done ON gas? (P=constant) System: Gas Surroundings: Piston, walls W W Gas P,V1,T1 Dy Isobaric process Gas P,V2,T2 W = -F s = -P A D y = -P DV DV > 0 expanding gas system does work on surroundings (isobaric process: pressure kept constant even though increase volume by adding heat – which offsets some but not all work done by gas) answer= negative work on gas What area is the total work done from 1 … to 4? P Isobaric Process: P=constant (horizontal line) Isochoric Process: V=constant (vertical line) P 1 W =- PDV (<0) P 1 2 V 2 1 P 1 V W =- PDV (>0) 2 4 3 DV < 0 V DV = 0 P 1 2 3 Wtot < 0 4 DV > 0 2 3 DV = 0 3 V W = -PDV = 0 4 2 3 W = -PDV = 0 4 3 Wtot = ?? 4 P 4 1 V V P 1 4 2 If we go the other way then 3 Wtot > 0 V Now try this: a. b. C. D. 1 to 2 to 3 to 1 (one cycle) What’s the total change in internal energy U? Is positive or negative work done? Is heat absorbed or released? Write an equation for work using P’s and V’s P P1 P3 1 2 3 V1 V2 V DU = 0 since no change overall (DT=0) overall net work is negative (clockwise) Heat is absorbed (Q>0) since work <0 and U =0 Area = (V2-V1)x(P1-P3)/2 Concept Question Shown in the picture below are the pressure versus volume graphs for two thermal processes, in each case moving a system from state A to state B along the straight line shown. In which case is the work done by the system the biggest? 1. Case 1 P(atm) P(atm) 2. Case 2 3. Same correct A B 4 2 4 A B 2 Case 1 3 Case 2 9 V(m3) 3 Net Work = area under P-V curve Area the same in both cases! 9 V(m3) First Law of Thermodynamics Example P 2 moles of monatomic ideal gas is taken P from state 1 to state 2 at constant pressure P=1000 Pa, where V1 =2m3 and V2 =3m3. Find T1, T2, DU, W, Q. 1 V1 1. P V1 = n R T1 T1 = P V1/(nR) = 120K 2 V2 V 2. P V2 = n RT2 T2 = P V2/(nR) = 180K 3. DU = (3/2) n R DT = 1500 J or DU = (3/2) P DV = 1500 J 4. W = -P DV = -1000 J (neg work done on gas) 5. Q = DU - W = 1500 J - -1000 J = 2500 J > 0 heat gained by gas First Law of Thermodynamics Example 2 moles of monatomic ideal gas is taken from state 1 to state 2 at constant volume V=2m3, where T1=120K and T2 =180K. Find Q. 1. P V1 = n R T1 P1 = n R T1/V = 1000 Pa 2. P V2 = n R T1 P2 = n R T2/V = 1500 Pa P P2 2 P1 1 V V 3. DU = (3/2) n R DT = 1500 J 4. W = -P DV = 0 J (no change in volume) 5. Q = DU - W = 1500 – 0 = 1500 J => It requires less heat to raise T at const. volume than at const. pressure. Example Sketch a PV diagram and find the work done on the gas during the following stages. (a) A gas is expanded from a volume of 1.0 L to 3.0 L at a constant pressure of 3.0 atm. W ON P D V 3 x10 ( 0 . 003 0 . 001 ) 5 (b) -600 J The gas is then cooled at a constant volume until the pressure falls to 2.0 atm W PDV 0 since D V 0 Example continued a) The gas is then compressed at a constant pressure of 2.0 atm from a volume of 3.0 L to 1.0 L. W ON P D V 2 x10 (. 001 . 003 ) 5 b) +400 J The gas is then heated until its pressure increases from 2.0 atm to 3.0 atm at a constant volume. W PDV 0 since D V 0 Example continued What is the net work ON gas? -600 J + 400 J = -200 J Rule of thumb: If the system rotates CW, the NET work is negative. If the system rotates CCW, the NET work is positive. Side note: work by gas = +200J NET work is the area inside the shape. Example A series of thermodynamic processes is shown in the pV-diagram. In process ab 150 J of heat is added to the system, and in process bd , 600J of heat is added. Fill in the chart. On gas. 150 0 150 J 600 -240 360 J 750 -240 510 J -90 600 0 600 -90 510 J Classification of Thermal Processes (using work on gas definition) Isobaric : P = constant Isochoric : V = constant: Isothermal : T = constant( DU = 0): Adiabatic : no heat flow (Q = 0) W = -P DV W=0 W = -Q W = - DU * Remember: work is area under P-V curve (positive work if compress gas to less volume) Thermodynamic Processes - Isothermal To keep the temperature constant both the pressure and volume change to compensate. (Volume goes up, pressure goes down) “BOYLES’ LAW” Same temp so DU=0 DU= Q + W= 0 so Q= -W DV = + since expands W = -PV = neg work Q= -W = positive heat added to maintain temp while gas uses own energy to expand to larger volume Thermodynamic Processes - Isobaric More heat Q is added to the gas than work energy used by the gas internal energy (U) decreases since PV is smaller. W= + (compress) Q= - release heat DU = - (colder at end) U= Q + W Q<W Thermodynamic Processes - Isovolumetric No work since constant volume W= 0 DU = Q Q = + since higher temp DU = + (higher int. energy) Thermodynamic Processes - Adiabatic ADIABATIC- (GREEK- adiabatos"impassable") In other words, NO HEAT can leave or enter the system. Q= 0 by definition U= Q + W U = W only W = - since gas expands hence U < 0 (tem drops) gas loses temp as expands since don’t provide heat to offset work done by gas In Summary Q=-W Q+W W Second Law of Thermodynamics “Heat will not flow spontaneously from a colder body to a warmer body AND heat energy cannot be transformed completely into mechanical work.” The bottom line: 1) Heat always flows from a hot body to a cold body 2) Nothing is 100% efficient Engines Heat flows from a HOT reservoir to a COLD reservoir Q H W QC W output Q H Q C QH = remove from, absorbs = hot QC= exhausts to, expels = cold Engine Efficiency In order to determine the thermal efficiency of an engine you have to look at how much ENERGY you get OUT based on how much you energy you take IN. In other words: e thermal W Q hot Q H QC QH 1 QC QH Rates of Energy Usage Sometimes it is useful to express the energy usage of an engine as a RATE. For example: The RATE at which heat is absorbed! The RATE at which heat is expelled. QH t QC t The RATE at which WORK is DONE W t POWER Efficiency in terms of rates e thermal QH W QH e QH t t QH t P t P W QC t P QH t Is there an IDEAL engine model? Our goal is to figure out just how efficient such a heat engine can be: what’s the most work we can possibly get for a given amount of fuel? The efficiency question was first posed—and solved—by Sadi Carnot in 1820, not long after steam engines had become efficient enough to begin replacing water wheels, at that time the main power sources for industry. Not surprisingly, perhaps, Carnot visualized the heat engine as a kind of water wheel in which heat (the “fluid”) dropped from a high temperature to a low temperature, losing “potential energy” which the engine turned into work done, just like a water wheel. Carnot Efficiency Carnot a believed that there was an absolute zero of temperature, from which he figured out that on being cooled to absolute zero, the fluid would give up all its heat energy. Therefore, if it falls only half way to absolute zero from its beginning temperature, it will give up half its heat, and an engine taking in heat at T and shedding it at ½T will be utilizing half the possible heat, and be 50% efficient. Picture a water wheel that takes in water at the top of a waterfall, but lets it out halfway down. So, the efficiency of an ideal engine operating between two temperatures will be equal to the fraction of the temperature drop towards absolute zero that the heat undergoes. Carnot Efficiency Carnot temperatures must be expressed in KELVIN!!!!!! The Carnot model has 4 parts •An Isothermal Expansion •An Adiabatic Expansion •An Isothermal Compression •An Adiabatic Compression The PV diagram in a way shows us that the ratio of the heats are symbolic to the ratio of the 2 temperatures Example A particular engine has a power output of 5000 W and an efficiency of 25%. If the engine expels 8000 J of heat in each cycle, find (a) the heat absorbed in each cycle and (b) the time for each cycle P 5000 W e 1 QC QH e 0 . 25 0 . 25 1 8000 QH Q c 8000 J Q H 10,667 J W Q H Q C W Q H 8000 W P 2667 J W t 5000 W t t 0.53 s Example The efficiency of a Carnot engine is 30%. The engine absorbs 800 J of heat per cycle from a hot temperature reservoir at 500 K. Determine (a) the heat expelled per cycle and (b) the temperature of the cold reservoir e W 0 . 30 QH W W 800 J W Q H Q C W 800 Q C QC 560 J eC 1 TC TC TH 350 K 0 . 30 1 TC 500 240 J