Lecture 11
advertisement

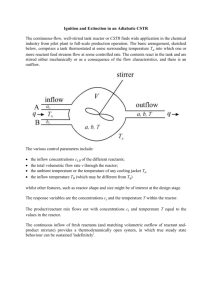
Lecture 11 Principles of Mass Balance Box Models: The modern view about what controls the composition of sea water Two main types of models used in chemical oceanography: • Box (or Reservoir) Models • Continuous Transport-Reaction Models In both cases: Change in Mass with Time Sum of Inputs Sum of Outputs At steady state the dissolved concentration (Mi) does not change with time: (dM/dt)ocn = ΣdMi / dt = 0 (i.e. the sum of the sources must equal the sum of the sinks at steady state) Change in Mass with Time = 0 Sum of Inputs Sum of Inputs Sum of Outputs Sum of Outputs How could we verify that this 1-Box Ocean is in steady state? For most elements in the ocean: (dM/dt)ocn = Fatm + Frivers - Fseds + Fhydrothermal If we assume steady state, and assume atmospheric flux is negligible (safe for most elements)… the main balance is even simpler: Frivers all elements = Fsediment all elements + Fhydrothermal source: Li, Rb, K, Ca, Fe, Mn sink: Mg, SO4, alkalinity Residence Time = mass / input or output flux = M / Q =M/S Q = input rate (e.g. moles y-1) S = output rate (e.g. moles y-1) [M] = total dissolved mass in the box (moles) d[M] / dt = Q – S Source = Q = = Sink = S e.g. river input flux Zeroth Order flux (flux is not proportional to how much M is present in the ocean) = many removal mechanisms are First Order (the flux is proportional to how much M is there) (e.g. radioactive decay, plankton uptake, adsorption by particles) First order removal is proportional to how much is there. S = k [M] where k (sometimes ) is the first order removal rate constant (t-1) and [M] is the total mass. Then, we can rewrite d[M]/dt = Q – S, to include the first order sink: d[M] / dt = Q – k [M] At steady state when d[M]/dt = 0, Q = k[M] Rearrange: [M]/Q = 1/k = * and [M] = Q / k *inverse relationship between first order removal constant and residence time Reactivity vs. Residence Time Cl sw Al, Fe Elements with small k have short residence times. When < sw the element is not evenly mixed! Dynamic Box Models In some instances, the source (Q) and sink (S) rates are not constant with time OR they may have been constant, but suddenly change. Examples: Glacial/Interglacial cycles, Anthropogenic Inputs to Ocean Assume that the initial amount of M at t = 0 is Mo. The initial mass balance equation is: dM/dt = Qo – So = Qo – k Mo The input increases to a new value Q1. The new balance at steady state is: dM/dt = Q1 – k M and the solution for the approach to the new equilibrium state is: M(t) = M1 – [(M1 – Mo)e-kt] “M increases from Mo to the new value of M1 (Q1/k) with a response time of k-1 or ” Dynamic Box Models M(t) = M1 – [(M1 – Mo)e-kt] = This response time is defined as the time it takes to reduce the imbalance (M1 – Mo). to e-1 (or 37%) of the initial imbalance ((1/e)*( M1 – Mo)). This response time-scale is referred to as the “e-folding time”. If we assume Mo = 0, after one residence time (t = ) we find that: Mt / M1 = (1 – e-1) = 0.63 This is 37% reduced, = e-folding time! For a single box model with a 1st order sink, response time = residence time. Elements with a short residence time will approach their new value faster than elements with long residence times. Introducing the 2-Box Model Mass balance for surface box Vs dCs/dCt = VrCr + VmCd – VmixCs – B At steady state: B = VrCr + VmixCd – VmixCs and fB= VrivCriv Broecker (1971) defines some parameters for the 2-box model Two important parameters are g and f: g = = = f = = the fraction of an element put in at the surface that is removed as B (the efficiency of bioremoval of an element from the surface – how efficiently it sinks as a particle (as B flux) out of the surface ocean) B / surface ocean input (VmixCD + VrCr – VmixCs) / VmixCd + VrCr the fraction of particles that are buried (the efficiency of ultimate removal from the water column) VrCr / B = VrCr / (VmixCd + VrCr - VmixCs)