Testicular cancer overview
advertisement

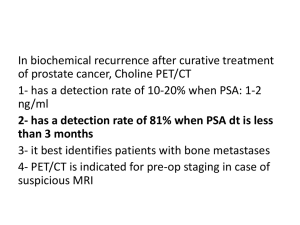
Biomarkers in Prostate Cancer, part II Prostate Cancer Symposium September 17, 2011 Clara Hwang, MD Internal Medicine Hematology/Oncology Biomarkers – the holy grail of personalized medicine? • Advances in technology (genomics, proteomics) have offered the promise of personalized medicine, where therapies and medical decision making can be finely tailored to patients. • Potential benefits include improving clinical efficacy and decreasing toxicity by better treatment selection and patient selection. Phases of biomarker development • Discovery – ~Phase I • Identify relationship between molecular marker and clinical outcome; ideally identify causal relationship • Analytic Validation – ~Phase II • Develop lab SOP/QC, reliability in clinical samples • Selectivity, sensitivity, CR, precision, accuracy • Clinical Qualification – ~Phase III • Confirm correlation with clinical outcomes • Clinical implementation - ~Phase IV Clinical utility of biomarkers in prostate cancer Clinical scenario Example Detection PSA for early diagnosis Prognosis Gleason score Prediction ER positivity and hormone Rx; none exist for prostate cancer Pharmacodynamic Testosterone for GnRH agonist Clinical surrogate PSA decline as tumor marker Prostate cancer clinical states and treatment decisions Localized disease Metastatic disease Should I start treatment? (Prognostic biomarker) Which treatment should I use? (Predictive biomarker) Is my treatment working? (Pharmacodynamic biomarker) Is my treatment helping the patient? (Surrogate biomarker) Current landscape of treatments for castrateresistant metastatic prostate cancer • Minimally symptomatic – sipuleucel-T • 1st line – docetaxel • 2nd line – • Abiraterone • Cabazitaxel • 3rd line – mitoxantrone Biomarkers to assess response to systemic therapy • Limitations of systemic therapy – all patients will ultimately progress • Some patients will not have an initial response to therapy • Are there predictive or surrogate biomarkers to guide treatment decisions? Response markers in prostate cancer • In current clinical use • • • • PSA Pain Bone scan CT scan/ MRI scan (RECIST) • Experimental • Circulating tumor cells The difficulty of assessing response to therapy in mCRPC “PCWG2 advises that, in the absence of clinically compelling indicators of disease progression, early changes (within 12 weeks) in indicators such as serum PSA, patient-reported pain, and radionuclide bone scan be ignored.” Scher et al JCO 2008 v26(7) p 1148 Clinical examples of using PSA as biomarker for treatment response • On occasion, PSA will rise prior to falling (PSA flare response) 140 120 100 % baseline PSA • PSA responses from three patients started on chemotherapy with CRPC 80 60 40 20 0 0 3 6 9 12 weeks 15 18 PSA response as surrogate marker • Retrospective analysis of patients on Ph III comparision of D+E vs M+P (S9916) • 3 month 30% PSA decline defined to have occurred if lowest PSA within first three months <=50% of baseline value • By this definition – occurred in 76% in D+E arm vs 40% in M+P Petrylak 2006 JNCI 98:516 PSA decline as surrogate marker in S9916 Original figure of MP vs. DE compared to OS curve of patients with PSA decrease >=30% in 3 mo vs < 30% Petrylak 2006 JNCI 98:516 Circulating tumor cells (CTC) • In patients with solid tumors, circulating tumor cells can be detected in circulation • Rare (1 in 109 nucleated cells) • Provide a source of tumor cells for molecular profiling • For Cellsearch assay, cutoff for favorable vs unfavorable is 5 CTC per 7.5 mL blood CTC detection with CellSearch Only FDA approved, analytically validated CTC assay Baseline CTC is prognostic in mCRPC patients treated with cytotoxic therapy de Bono J S et al. Clin Cancer Res 2008;14:6302-6309 Conversion of CTC as predictor of overall survival de Bono J S et al. Clin Cancer Res 2008;14:6302-6309 CTC enumeration vs PSA decline as predictor of overall survival Clinical utility of biomarkers in prostate cancer, revisited Clinical scenario Example Detection PSA for early diagnosis Prognosis Gleason score Prediction ER positivity and hormone Rx; none exist for prostate cancer Pharmacodynamic Testosterone for GnRH agonist Clinical surrogate PSA decline as tumor marker Markers that correlate with docetaxelsensitivity underexpressed in sensitive cell lines overexpressed in sensitive cell lines Causal relationship between SKP2 and docetaxel response Effect of SKP2 knockdown on docetaxel response si-SKP2 100 0.8 0.6 0.4 0.2 0.0 LNCAP Cell viability, % baseline Relative Quantitation si-nontarget 1.0 80 60 40 Actin SKP2 1 10 docetaxel concentration (log scale) 100 Conclusions • PSA decline may be used as a surrogate marker for response (after 12 weeks) • Baseline levels of CTC are prognostic for OS • Conversion of CTC correlates with OS but have not yet been qualified for individual patient use • Further studies are needed to identify and validate predictive markers