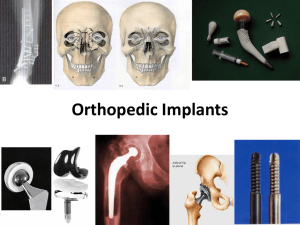
Orthopedic Implants
advertisement

Schematic illustration of the various fixation methods: (a) direct interference or (passive) noninterference fit; (b) mechanical fixation using screws, bolts, nuts, wires, etc.; (c) bone cement; (d) porous ingrowth (biological) fixation; (e) direct chemical bonding using adhesives or after coating with direct bonding material layer; (f) bone cement with resorbable particles; (g) porous ingrowth controlled by using electrical or electromagnetic stimulation Bone Cement Polymethylmethacrylate (PMMA) bone cement: – made from methylmethacrylate, polymethylmethacrylate, esters of methacrylic acid, or copolymers containing polymethylmethacrylate and polystyrene • Companies: – Johnson & Johnson, Stryker, Zimmer, Smith & Nephew, Exactech, Heraeus Holding, Biomet Examples of currently used surface coatings on stems of THA to enhance both short- and long-term fixation عوارض احتمالي تعویض مفصل ران • • • • • ایجاد لخته هاي خون (داروهاي رقيق كننده ي خون ،جوراب كشسان ،انجام ورزشهايي كه جريان خون را به عضالت پا افزايش دهند ،استفاده ازچكمه هاي پالستيكي كه براي تحت فشار قرار دادن عضالت پا،با هوا باد مي شوند. شل شدگي پروتز دررفتگي گوي از كاسه (جا انداختن ،استفاده از آتل) ساييدگي آسيب به عصب Approximate Average Concentrations (ng/ml or ppb) of Metal in Human Body Fluids with and without Total Joint Replacements Bone Void Fillers A resorbable calcium salt bone void filler device is a resorbable implant intended to fill bony voids or gaps of the extremities (leg & hand), spine, and pelvis that are caused by trauma or surgery • Homework: Find different types of bone fillers? Knee degrees of freedom • • • • Flexion – ±50° IE Rotation – ±25 ° AP Translation - ±25 mm Axial load – 4500 N Total Knee Replacement -Partial knee replacement (unicompartmental) – Replacement of one or two parts of knee instead of total. – Retain more of patient natural knee. • Total knee replacement – Resurfaces the bones (tibia and femur) with an implant made of metal and plastic parts. – Medical-grade plastic spacer providing a smooth surface as cartilage The time of TKR Step I: – If patients answer yes to any of the questions, it may be time to speak with an orthopedic surgeon about knee replacement surgery 1. Does the knee hurt one or more days per week?___Yes___No 2. Does the pain interfere with sleep? ___Yes___No 3. Is it painful to walk more than a block? ___Yes ___No 4. Are pain medications no longer working? ___Yes___No 5. Is knee pain limiting participation in activities? ___Yes___No 6. Has inactivity from knee pain caused weight gain? ___Yes___No 7. Have you ever received hyaluronic acid injections? ___Yes___No 8. Would it be possible to limit activities for a few months to recover from surgery? ___Yes___No 9. Are you willing to commit to work hard during rehabilitation for a successful recovery? ___Yes___No Knee joints FDA Approved • Knee joint femorotibial metallic constrained cemented prosthesis • Knee joint femorotibial metal/composite non-constrained cemented prosthesis • Knee joint femorotibial metal/polymer constrained cemented Examples of new THA and TKA oxidized zirconium components currently gaining popularity because of enhanced mechanical and biocompatibility properties Spinal fixation device Ankle Joint Replacement Ankle Joint Replacement Some ankle prostheses FDA Approved • Metal/composite cemented semi-constrained ankle prosthesis • Metal/polymer cemented semi-constrained ankle prosthesis • Non-constrained cemented ankle prosthesis – Co-Cr alloy and UHMWPE or UHMWPE –carbon fiber • Companies: Johnson & Johnson, Stryker, Biomet, wright medical technology, Tornier Shoulder Joint Replacement Shoulder Joint Prosthesis Shoulder joints FDA Approved • Shoulder joint metal/metal or metal/polymer constrained cemented prosthesis • Shoulder joint metal/polymer semi-constrained cemented prosthesis • Shoulder joint metal/polymer non-constrained cemented prosthesis • Shoulder joint metal/polymer/metal nonconstrained or semi-constrained porous-coated uncemented • Shoulder joint glenoid (hemi-shoulder) metallic cemented prosthesis • Humeral Metal/Polymer Cemented Or Uncemented Elbow Joint Prosthesis Wrist Joint Prosthesis FDA Approved • Wrist joint carpal lunate polymer prosthesis • Wrist joint carpal scaphoid polymer prosthesis • Wrist joint carpal trapezium polymer prosthesis • Wrist joint polymer constrained prosthesis Proximal: A=Scaphoid, B=Lunate, C=Triquetral, D=Pisiform Distal: E=Trapezium, F=Trapezoid, G=Capitate, H=Hamate Finger joint prostheses Finger joint prostheses FDA Approved • Phalangeal (hemi- toe) prosthesis – Made of silicone elastomer – Replace the base of the phalanx of the toe – Companies: Wright medical technology, Arthrex, Ascension orthopedics, Dow Corning • Polymer constrained toe prosthesis – Made of silicon or polyester reinforced silicon – To replace the first metatarsophalangeal (big toe) joint – Companies: Biomet, Smith & Nephew, Zimmer holdings, Dow corning • Semi-Constrained Metal/Polymer Joint Toe (Metatarsophalangeal) Prosthesis – Companies: Ascension Orthopedics, Biomet, Acumed Laminoplasty A surgical procedure for treating spinal stenosis by relieving pressure on the spinal cord • The MOUNTAINEER Laminoplasty System is a complete set of implants and instruments designed to allow for a systematic approach to laminoplasty procedures in the cervical spine. Stenosis = abnormal narrowing of a bodily canal or passageway Cartilage injuries • Disease, accidents and age • Limited repair capability: avascular • Differences in the types of cartilage lesions • No absolutely approved and perfect method • Tissue engineering as a new treatment method based on cells, scaffolds and growth factors Methods for Articular Cartilage Repair or Regeneration Limitations • Needs two surgeries • Ages 15 to 45 • Defect size (6mm×7mm) • BMI < 30 • Not used in the presence of osteoarthritis, inflammatory disease, rheumatoid arthritis • Not used for patients whose knee meniscus has been surgically removed Application of natural biomaterials Commonly Used Scaffolds in Autologous Chondrocyte Implantation (even distribution and settlement of cells in the defect) Injections for Knee Pains • Corticosteroid injections for osteoarthritis as an antiinflammatory treatment • Hyaluronic acid injections as viscosupplementation – Exercise and simple pain medications no longer effectively manage knee pain caused by oseoarthritis – Injections reintroduce healthy joint fluid – Injections restores cushioning and lubricating properties to the knee joint • Orthovisc® (high molecular weight hyaluronan) • FDA approved non-drug therapy • Made from ultra-pure natural hyaluronan, a naturally occuring lubricant found in healthy knee joints • Provide up to six months of pain relief Meniscectomy • Remove the damaged portion of the meniscus – Starts by making several small incisions in the knee, and insertion a very small video camera called an arthroscope – The surgeon watches on a video screen while probing the meniscus – Surgical instruments are used to remove the torn portion of meniscus – A small motorized cutter is used to trim and shape the cut edge of the meniscus – The joint is flushed with sterile saline to wash away debris from the injury or from the surgery – The entrances are closed with sutures • Tip: – Special fasteners, called suture anchors, are sometimes used to anchor the torn edges of the meniscus together for younger patients PTFE Ligament Prosthesis Ligament Repair • A graft taken either from the individual with the injury or a donor • Small incisions for insertion an arthroscope to determine the extent of the injury the appropriate surgical procedure • The torn pieces of the ligament are removed • The pathway for the new ligament is prepared • A guide wire is drilled in the thighbone (femur) • A drill is placed over the guide wire and a hole equal to the graft size is drilled through the bone • A stitch is connected from the femur to a guide wire and this is pulled through predrilled holes in the thighbone and shinbone • Fixation of the surgeons choice is then placed in the thighbone • The new ligament graft is then tensioned and a second method of fixation is placed in the shinbone to secure the new ligament to the surrounding bone • After reconstructing the ligament, the portals are closed with stitches or surgical tape Fibrin sealants Production of a fibrin clot as an adhesive and wound-covering agent • In synthetic process the fibrinogen is at a much higher concentration (70 mg/ml) than that in human plasma • After mixing fibrinogen polymerized to fibrin monomers and A white fibrin clot is initiated under the action of thrombin And CaCl2 • Aprotinin, an inhibitor of fibrinolysis, may also be Included in solution A Advantages of Fibrin It is hemostatic • It adheres to connective tissue • It promotes wound healing • It is biodegradable with excellent tissue tolerance