Lecture 6 Powerpoint - McCausland Center | Brain Imaging
advertisement
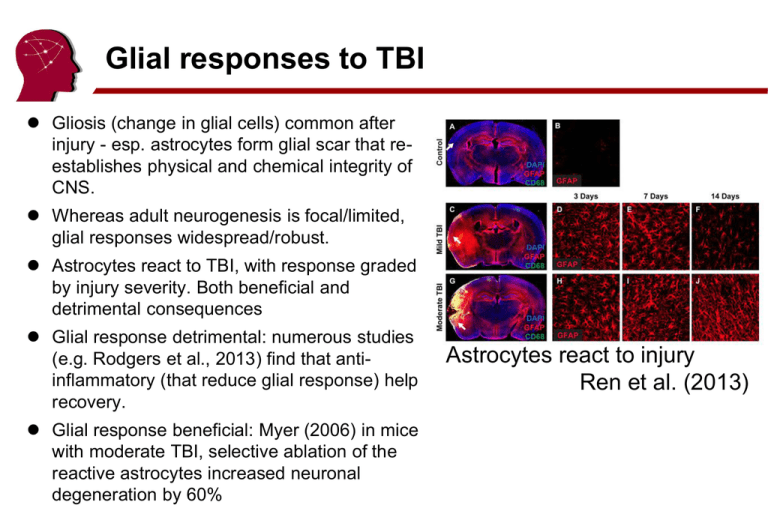
Glial responses to TBI Gliosis (change in glial cells) common after injury - esp. astrocytes form glial scar that reestablishes physical and chemical integrity of CNS. Whereas adult neurogenesis is focal/limited, glial responses widespread/robust. Astrocytes react to TBI, with response graded by injury severity. Both beneficial and detrimental consequences Glial response detrimental: numerous studies (e.g. Rodgers et al., 2013) find that antiinflammatory (that reduce glial response) help recovery. Glial response beneficial: Myer (2006) in mice with moderate TBI, selective ablation of the reactive astrocytes increased neuronal degeneration by 60% Astrocytes react to injury Ren et al. (2013) Concussions and permanent injury Some suggest the mildest TBIs cause no permanent injury: – American Association of Neurological Surgeons: “experts emphasize that although some concussions are less serious than others, there is no such thing as a ‘minor concussion’. In most cases, a single concussion should not cause permanent damage” – On the other hand, even if symptoms typically ~7 days, unclear if there is not subtle permanent injury. If no lasting injury, what is the mechanism for transient cognitive dysfunction? – Can this explain vulnerability for 2nd injury? – Popular models suggest all TBIs cause chemical cascade, this can either resolve or lead to permanent 2nd injury. Lifestyle: Football and dementia Savica et al. (2012) no significant difference between high school football (‘46-’56) and band Football (438) Lehman et al. (2012) 3439 NFL players from ‘59-’88. AD/ALS x4 expected, esp. Speed. Band (140) Speed (173) NonSpeed (152) Dementia 3.0% 1.4% Dementia 3.4% 0.6% Parkinson's 2.3% 3.6% Parkinson's 3.4% 0.6% ALS 0.7% ALS 0.6% 0.5% 1.1% Vanacore (2013): not necessarily due to TBI: perhaps intense physical activity, use of drugs, exposure to neurotoxins Biochemical changes in TBI Concussion (mTBI) lead to behavioral changes that typically resolve ~7 days (discussed last week) Understanding mechanism is important – Biomarkers for return to play – Are all concussions permanent TBI, or are some simply transient biochemical imbalances? – Might help treatment (prevent 2nd injury) Neuron Chemistry Neurons firing via electro-chemical signals. Requires energy gradient to work. Potassium/Sodium pumps expel two sodium and intake two calcium ions. Net result, neuron rests at -70mV 70% of the energy is used to maintain the Na+, K+ membrane potentials. Pumps work continuously to keep gradients, firing causes rapid, large brief expense of energy. When cell fires: sodium channels briefly open: sodium rushes in: neuron briefly has positive voltage (+30mV) next, potassium channels briefly open, potassium rushes out, voltage restored Refractory period Absolute: Neuron can not re-fire while sodium channels open Relative: Low sensitivity while potassium channels open K+ K+ Na+ Na+ Na+ Cellular Respiration Fueling neurons (can not use free fatty acids) Create pyruvate – Anaerobic Glycolysis (Primary) Convert glucose to pyruvate and 2 ATP Occurs in Cytoplasm – Ketone bodies (starvation, worst-case) Use Pyruvate – Aerobic (‘Conversion’, sustained, primary): Where: Mitochondria Efficient (36 ATP) Requires: O2 Waste: CO2, free radicals (specifically, reactive oxygen species [ROS]) Anti-oxidant enzymes to counteract free radicals – Anaerobic (‘Fermentation’, burst, backup) Where: Cytoplasm Inefficient (2 ATP) Waste: Lactic Acid (marker for fermentation) Metabolism Human Brain about 2% of body weight, 20% of energy. – Brain: glucose is principal fuel, ketone is emergency backup. – Rest of body: glucose or fatty acids. – Humans have relatively large brain/body (high energy demands) with large energy reserves (fat). – Other animals: ~5% energy at rest When fasting (or extremely low carb diet): – <0.25 days: glycogen reserves – 0.25..3 days: fat converted to free fatty acids and glucose – >3 days: liver converts fatty acids to ketones (@4 days, 70% of brain metabolism is from ketones). Even so, not enough glucose comes from fat breakdown, so additional glucose must come from breakdown of proteins (e.g. muscle). – Other mammals: Fat breakdown typically provides sufficient amount of glucose. No need to produce ketones or break down proteins. – Ketogenic diet controls pediatric epilepsy, potentially because calorie restriction, fewer free radicals, acidic (blocks ion channels), less glucose, more inhibitory GABA. Understanding 2nd Injury Barone & Feuerstein (1999) [for ischemic stroke] Necrosis: cell death - contusion and border, hippocampus Apoptosis: cell suicide - not due to membrane failure, glutamate mediated, minimal immune response Neurometabolic Cascade Giza & Hovda (2001) Sequence of biochemical changes after TBI. – Initial (minutes) K+ increase Glutamate release Lactate increase – Sustained (days) Blood flow (CBF) decrease Ca+ increase – Biphasic cerebral metabolic rate (CMR) of glucose initially increased, then decreased Why is glucose usage reduced? Clear evidence TBI leads to chemical changes Mechanism debated. Two (not mutually exclusive) options: – Decreased need for glucose metabolism. E.G. Pappius (1995) suggest noradrenergic and serotonergic reductions. However, these effects appear short lived (hours), whereas CBF disturbed for days. – Metabolic dysfunction, e.g. Giza & Hovda’s neurometabolic cascade Neurometabolic Cascade Giza & Hovda (2001) Model to explain sequence of Neurometabolic Cascade. – Initial injury: membranes injured, glutamate and K+ leak into extracellular space. – Ionic pumps work overtime K+, exhausting ATP. – Glutamate causes influx of calcium. – Glucose uptake initially increases, but Calcium overload makes aerobic conversion inefficient. – Claim: mTBI is primarily cellular dysfunction and little cell death, where in more severe TBI calcium leads to apoptosis. Neurometabolic Cascade Giza & Hovda (2001); Barkhoudarian, Hovda, Giza. (2011) 1. Cell membrane deformed/leaky: Glutamate released, Ca+ influx 2. Glutamate activates post-synaptic NMDA, Releasing K+, Intake Ca+ 3. Ionic pumps attempt to restore gradient (pump in K+, pump out Na+). This requires ATP 4. ~30 minutes: Hyperglycosis to create ATP 5. ATP demands lead to Lactate accumulation and Ca+ influx of mitochondria. 6. 30min-5days, mitochondria function inefficient: decreased glucose metabolism (50%) decreased aerobic activity, increased free radicals. Neurometabolic Cascade Barkhoudarian et al. (2011) – hyperglycolysis and oxidative dysfunction ~30 min post injury. Anaerobic glycolysis is the transformation of glucose to pyruvate when limited amounts of oxygen (O2) are available Inefficient anaerobic function designed for short bursts limited reserves. – ~6hrs post injury: glucose hypometabolism (approximately 50%). Lasts ~5days (mild) ~months (severe) – Tissue vulnerable to subsequent injury during this period – In mild cases cells eventually return to normal function, in severe cases apoptosis. Human evidence for metabolic cascade Vespa et al. (2005) Often small primary injury includes widespread dysfunction as observed by decreased oxidative metabolism (CMRO2) and altered glucose metabolism. Little Post-traumatic ischemia Ongoing metabolic crisis (elevated lactate/pyruvate ratio, LPR) Found LPR (anaerobic) negatively correlated with CMRO2 (aerobic) Human evidence – Stein et al. (2012) evaluated 72 individuals with controlled ICP during initial 72h of severe TBI. 76% Low glucose 93% elevated lactate/pyruvate ratio (LPR) >25 • Lactate produced by pyruvate only under anaerobic conditions 74% metabolic crisis (both low glucose and high LPR) Metabolic crisis predicted poor 6 month outcome Cascade Implications Cascade model suggests glucose conversion transiently disrupted. Switching to ketones when glucose use is disrupted may be beneficial. Recent studies suggest ketone neuroprotective (Prins, 2008) and post-injury (Prins et al., 2005; Deng-Bryant et al., 2011) in younger rats. Perhaps fasting/ketogenic diet useful for mTBI Cascade Summary Barkhoudarian et al. (2011) Implications – Return to play: "concussion-induced pathophysiologic conditions, as manifested by metabolic perturbations, altered blood flow, axonal injury, and abnormal neural activation, reduce cerebral performance and make the brain more susceptible to cellular injury” – Appears to suggest that some mTBI may be transient biochemical imbalance rather than permanent injury. Vulnerable window Prins et al. (2013) Glucose metabolism altered in rats for ~7 days (differs with age and injury). 2nd injury in this timeframe will have severe consequences Why? Perhaps poor auto regulation since CBF and metabolism uncoupled. Evidence for vulnerable period Humans: initially vulnerable to 2nd TBI – Is this biochemical or psychological (poor awareness)? Recent animal studies suggest ~7day window of vulnerability for 2nd injury. – Mice: Longhi et al (2005) – Rats: Vagnozzi et al. (2007) Tavazzi et al. (2007) – This data proves biochemical involvement Axon disconnection Park et al. (2008): In addition to damage to gray matter, specific secondary effects influence axonal disconnection 1. Impaired microcirculation due to stenosis and 2. astrocyte foot swelling 3. Proliferation of glial cells 4. Excess glutamate 5. Calcium Influx 6. Excitotoxicity 7. Ca accumulation 8. Disconnection Pause Break Predictors of TBI prognosis Considerations – Severity/Mechanism – Pre-injury function – Age – Health – Gender – Genetics (APoE e4) Resources – http://mitbitraining.org – http://www.nctbitraining.org/main.aspx TBI Classification Mechanism – Closed vs. Open Open: Penetrating vs. Perforating Pathology – General: Primary vs. Secondary Injury – Blast: Primary : Secondary : Tertiary : Quaternary Morphology – Focal vs. Diffuse Severity – Mild vs. Moderate vs. Severe Causes of TBI Civilian TBI causes varies with age Initial severity and outcome Dikmen et al. (1995) Relative to general trauma controls, TBI associated with poor cognitive performance – In particular: attention, memory, processing speed – At 1 year post injury, those with less than 1 hr TFC performed similar to controls, 1-24hrs impaired in attention and memory, longer had more global impairments. Zatick et al. (2011) also showed worse injuries associated with worse outcome and better recovery for milder deficits. Roozenbeek (2012): 39274 patients: age, GCS motor score, and pupillary reactivity strongly predict 6mos outcome. Predictors of good outcome More education is correlated with good prognosis (Kreutzer et al., 1993) and premorbid cognitive function (Hanks et al., 1999). – Perhaps due to cognitive reserve (Satz et al., 1993; Stern et al., 2006) Generally, severe symptoms predict poor outcome – Low GCS, long PTA and LOC, brain imaging findings, dural penetration, pupillary abnormalities, hypoxia, systemic complications – Acute neuropsychological results is a better predictor than neurological severity (Hanks et al., 2008) Recovery from mild TBI Numerous studies suggest mild TBI typically resolves <3 months without treatment (Dikmen et al., 1986; Dikmen et al., 2001; Levin et al., 1987; Barth et al., 1989; Macciocchi et al., 1996; McCrea et al. 2003) – 90% spontaneous recovery – 10% persistent symptoms, include dizziness, headaches, pain, fatigue, depression, return to work. If complicated mTBI, ‘post concussive syndrome’. Recovery from Moderate/Severe TBI Millis et al. (2001) tracked 182 individuals 5 years post injury – 22% improved, 63% unchanged, 15% declined – Improvement in processing speed, visuoconstruction, verbal memory Dikmen et al. (1995) 1yr post injury, more severe TBI were 25 percentile points lower than trauma controls. Salmond et al. (2005) more severe TBI impaired in attention, verbal learning & reaction time, but spared spatial working memory. Not all cognitive deficits are organic: medication, depression and premorbid factors contribute. Gender TBI more frequent in men then women Iverson et al. (2011) study of 11951 men and 654 women with TBI from Afghanistan/Iraq – PTSD most common, women relatively less – Depression common, women x2 more – Anxiety disorders, women x1.3 more – Type of TBI (e.g. blast) might explain some of these differences Genetics APoE 4 associated with Alzheimers Disease (independent of TBI). APoE 4 and recurrent TBI have cognitive and dementia risks (see sports literature: Jordan et al., 1997; Kutner et al., 2000). Impact on single TBI remains controversial – Teasdale (2005). Large study (1094 patients, 513 with mTBI) showed no difference at 6 mos. However, potential interaction such that pediatric TBI with APoE 4 had worse outcome Pediatric TBI Giza (2006) review notes differences – Pediatric skull thinner, more pliable – Larger head relative to body, less developed neck muscles – Different causes (pediatric falls) – Higher blood flow, higher metabolism – Higher incidence of post traumatic epilepsy – Interfere with developmental potential – Calcium influx more diffuse in children (see cascade slides) – Young brain more vulnerable to excitotoxic injury Treatment: education of caregivers (parents, teachers); continuous assessment (skills may not have yet developed). Elderly TBI Roe et al. (2013) examined severe Norwegian TBI in adults vs elderly. – Elderly more likely to have suffered falls – Hematoma more common Dura sticks to skull, anticoagulants common – 25% of adults and 66% of elderly died within 3 mos. – Adults more likely to be inpatients and go to rehab units. – No difference in functional outcome at 3 mos. Clinically: Work rehab not as important, since there is less recovery, educate caregivers. Pause Break Military Traumatic Brain Injury – Brain related problems major issue for military Incidence and Financial Costs Reasons for high incidence Blast Induced Neuro Trauma Gunshot wounds – Resources http://www.dvbic.org/resources http://www.gvsu.edu/veteranstbi/ ($) War related injuries In Vietnam, wounded: killed was 2.6:1 In Iraq/Afghanistan the ratio is 16:1 – Radically improved acute care Embedded medics New training and medical equipment • decompressive craniotomy common for evacuation (ICP) New body armor Different type of injury (in Iraq/Afghanistan, typically blast) Low rate of injury means ability to provide maximal acute care (e.g. no triage) – Clear long-term obligations for care US Health Care Costs US spends disproportionate amount on health care. Yet, 48m US citizens do not have health care Rising cost of military medicine DoD health care costs rose from $19bn in 2001 to $49.4bn in 2014. VA 2014 Budget rose from $48.7bn in 2001 to $152.7 billion in 2014 VA Budget Mental Disorders in US Military Mental disorders largest and fastest growing military hospitalization Not only TBI: 20% from Iraq/Afghanistan report PTSD/depression. Military TBI Higher incidence (better awareness?) Incidence by severity Increase appears to be in mild TBI – Better identification? – Are ‘mild’ blast TBIs similar to other mild TBIs? Military TBI approximately 80% of service members TBIs occur in a nondeployed setting. Common causes of TBI include vehicle crashes, falls, sports and recreation activities, and military training. 77% mild TBI 3.2% Civilian vs Military TBI Blast TBI signature injury from current wars. Civilian Causes Vary With Age 68% injured in combat have blast injuries TBI in the military TBI incidence for 1.6m deployed to Afghanistan/Iraq – ~1800 penetrating wounds. – 66% of all wounded soldiers that do not return immediately to service have TBIs. Total combat TBI rates vary between sources – ~10,000 blast injuries (assuming 85% have closed head injuries) – ~30% of troops who have been at the front for >4 months at risk from blast injury. Blast Induced Neuro Trauma Blast leading cause of injury/death in Iraq – 69.4% of wounded caused by explosion – 62% of blast injuries result in TBI – 85% of TBIs are closed head Blast Injury : Multiple types of TBI Blast Injury Primary Blast Injury: blast pressure wave[s] Secondary Blast Injury: Sharpnel stiking victim – e.g. penetrating injury Tertiary Blast Injury: Victim hitting objects – Closed or open head injury Quaternary Blast Injury: Other – Include flash burns, crush and respiratory injuries, psychological consequences NB: Primary, Secondary and Tertiary Blast Injuries can each cause primary or secondary brian injuries. BINT Consequences Cernak and Noble-Haeusslein (2010): Despite similar secondary injury cascades, BINT has characteristics not seen in other types of brain injury – weight loss – hormone imbalance – chronic fatigue – headache – problems in memory, speech and balance Blast injuries MacDonald et al. (2011) examined 21 controls and 63 soldiers with blast related mTBI (no injury detected with CT), DTI within 90 days of injury. (47 followed up 6-12 months later) – Reduced white matter near Cerebellum, Cingulate, Orbitofrontal cortex. Blast Induced Neurotrauma (BINT) BINT signature injury of recent wars Overpressure has dramatic effects on gascontaining organs (lungs, ears). Brain mostly liquid/solid and not compressible (Monro-Kellie doctrine). – Both initial pressure wave and reflections can cause injury. Scott et al. (2006): Military blast associated with Hearing loss (42%), eye injuries (26%), brain injuries (66%), abdominal injuries (22%) and stress syndromes. Blast injury Lu et al. (2012) exposed monkeys to blast injuries. Histology at 3 days or 1 mos post injury – Behaviorally: working memory and motor impairments – Purkinje neurons in the cerebellum and pyramidal neurons in the hippocampus – White matter injury to myelinated axons. Apoptosis of astrocytes. Return to duty Scherer et al. (2013) describe current and suggested guidelines for ‘tactical athletes’ based largely from knowledge of sports – Classic concussion “duty restrictions for at least 24 hours, longer if symptoms persist”. – Recently, once asymptomatic, exercise to target heart rate and then conduct cognitive task (Exertion to 65%-85% of predicted heart rate maximum). – Take into account symptoms, comorbidities, history (previous exposure). Posttraumatic epilepsy TBI-related epilepsy accounts for 20% of symptomatic (cause known) epilepsy, 5% of all epilepsy (mostly idiopathic [cause unknown], often with genetic component). Garga and Lowenstein 2006 review suggests 2-25% incidence depending on TBI severity Salazar et al. (1985) found 53% incidence of epilepsy in 421 Vietnam vets with penetrating brain wounds. Seizures often months to years after injury. Ballistic Trauma (Gunshot wounds) GSW often cause widespread white matter shearing.









