: Intermittent Neurogenic Claudication
Aperius® Percutaneous Interspinous Spacer
F. Collignon, P. Fransen, D Morelli, N. Craig, J. Van Meirhaeghe
For the INCA-study investigator group
Disclosure information
Patrick Fransen
Study design
STUDY DESIGN
International, multi-center, single arm trial
N° PATIENTS
162 patients consented, 157 patients treated
CLINICAL SITES
12 European: 6 in Belgium, 5 in Germany, 1 in United Kingdom
FOLLOW-UP
48 hours, 7 days, 6 weeks, 6 months and 12 months
ENDPOINTS
Primary
Safety: procedural or device related SAE < 7 days
Effectiveness: ZCQ-Symptom Severity at 6 weeks
Secondary
Safety: procedural or device related SAE in follow-up
Effectiveness: ZCQ Symptom Severity
ZCQ Physical Function and EQ-5D
Radiological
Implant status & positioning, change in lordosis, p-MRI,
maintenance of distraction
PURPOSE
Evaluate safety of the procedure and the safety and effectiveness of
Aperius® Percutaneous Interspinous Spacer
Patient Demographics & Surgical Procedure Information
Sex
50.3% Male
49.7% Female
Mean age
64,9 years ± 11,9
Mean complaint duration
40.0 months ± 60,4
Treatment history
Pain medication : 85,4 %
Physical therapy : 66,2 %
Epidural infiltration : 59,2 %
N° of stenotic levels
1 level : 69 patients
2 levels : 78 patients
3 levels : 10 patients
Treated levels
L1-L2 : 0.4 %
L2-L3 : 5.9 %
L3-L4 : 35.5 %
L4-L5 : 58.2 %
Device size used (n=250)
8mm : 6.4 %
10mm : 24.8 %
12mm : 39.2 %
14mm : 29.6 %
Mean estimated procedure time
(skin to skin)
One level : 15.5 min ± 9.1
Two levels : 24.6 min ± 8.6
Three levels : 39.1 min ± 8.9
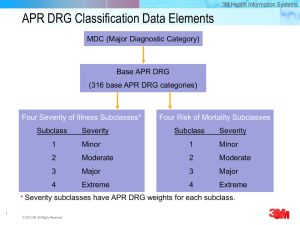
Primary Effectiveness Endpoint
Zurich Claudication Questionnaire on Symptom Severity
5
Primary endpoint
4
3
*
2
*
*
*
3
2.1
2.1
2.2
2.2
7 days
(n=156)
6 weeks
(n=155)
6 months
(n=151)
12 months
(n=155)
1
Baseline
(n=157)
At 6 weeks the mean ZCQ Symptom Severity was reduced by 29.7%.
This statistical significant improvement (*p<0.001) was maintained at 6 and 12 months
Secondary Effectiveness Endpoints
ZCQ-Physical Function
VAS Pain Scores
10
4
9
8
*
7
3
*
*
*
2
*
*
*
6
5
Leg pain
4
Buttock/groin pain
Back pain
3
2,5
2,3
1,8
1
1,9
1,8
2
1
0
BL
(n=157)
7d
(n=155)
6w
(n=155)
6m
(n=149)
BL
12m
(n=153)
Max. improvement (0.7 points in ZCQ PF) reached at
6 weeks (*paired t-test p<0.001)
7 days 6 w eeks
6m
12m
Immediate significant pain reduction and at all
timepoints compared to baseline (*p<0.001)
Pain medication
Walking distance
100,0%
100%
80%
Morphine
80,0%
>1000
Central acting (Tramadol)
60%
Codeine combinations
NSAID
40%
60,0%
500-1000
100-500
40,0%
<100
Paracetamol and/or aspirine
No pain medication
20%
20,0%
0,0%
0%
Screening
7 d ays
6 weeks
6 mo nt hs
12 mo nt hs
The use and strenght of pain medication used
decreased over time
Screening
(n=157)
7 days
(n=156)
6 weeks
(n=156)
6 months
(n=153)
12 months
(n=156)
Walking distance improved overall and was
maintained over total follow-up period
Safety
Primary safety endpoint - First 7 days post-operatively
•
6 serious adverse device related effects (SADE) were observed
• One patient with a spinous process fracture
• Three patients suffering from back pain
• One hematoma
• One patient experiencing pain in the lower extremities
Overall safety assessment - Up to 12 months
•
11 patients experienced an SADE, mainly reporting back pain and recurrence of NIC symptoms
•
52 patients experienced an ADE of which 26 reported as back pain and 10 recurrent NIC
symptoms.
•
14 explant procedures were performed while 90.7% of the population had their device still in place
•
Nine confirmed cases of spinous process fracture were observed during the study, 3
spontaneously reported cases and 6 cases confirmed following central reading of X-rays. The
clinical outcome of all nine patients was analysed but no correlation was found.
Conclusion
1.
2.
The procedure was shown to be generally safe and well tolerated
•
11 patients experienced SADEs during the total follow-up period
•
No unexpected ADEs or SADEs were identified
The device was effective for up to 12 months post-operatively when used for the relief of
NIC complaints in patients with symptomatic DLSS
•
3.
Statistical significant improvement from baseline was shown in:
•
both symptom severity and physical function (ZCQ)
•
leg, back and buttock/groin pain assessed by VAS
•
Quality of life assessed by EQ5D
•
A reduced use of pain medication was reported.
•
Significant improvement in walking distance was observed.
The APERIUS® device offers advantages due to the percutaneous approach
•
Short operation time
•
Potential use of local anesthesia
•
Minimal/negligible blood loss
•
Short recovery and rehabilitation period