Integrating Radiotherapy Trials QA into NCRI Clini
advertisement

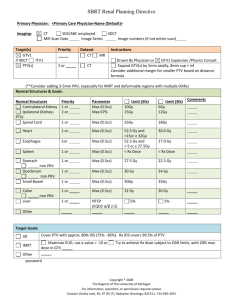
Integrating Radiotherapy Trials Quality Assurance (RTTQA) into National Cancer Research Institute (NCRI) clinical trials Lisette Nixon Senior Trial Manager Wales Cancer Trials Unit Cardiff University 1 Co-ordinator Cardiff RTTQA group Velindre Cancer Centre 2 Lucy Wills 2, Emiliano Spezi 2, Sarah Gwynne 2, Rhydian Maggs 2, Tony Millin 2, Chris Hurt 1, Geraint Lewis 2, John Staffurth 2 , Gareth Griffiths 1 1 Wales Cancer Trials Unit, Cardiff University, 6th Floor, Neuadd Meirionnydd, Heath Park, Cardiff CF14 4YS 2 Velindre Cancer Centre, Whitchurch, Cardiff, CF14 2TL Who’ who Wales Cancer Trials Unit (WCTU) – NCRI accredited Clinical Trials Unit who develop and run cancer clinical trials National Cancer Research Institute – UK-wide partnership between the government, charity and industry which promotes co-operation in cancer research Radiotherapy Trials Quality Assurance Group (RTTQA) – NCRI Group formed to provide central QA and advice for all RT trials RTTQA Cardiff – Sub-group of the main RTTQA group Velindre NHS Trust – NHS Trust within which the Cardiff RTTQA group sit and also sponsor for the SCOPE 1 trial What is Radiotherapy? http://www.oncoprof.net/Generale2000/g08 _Radiotherapie/gb08_rt06.html http://cancerhelp.cancerresearchuk.org/type/wombcancer/treatment/radiotherapy/external-radiotherapy-for-womb-cancer Planning of Radiotherapy Patient has a planning CT scan in treatment position Clinician uses diagnostic information to draw round the tumour (GTV: Gross Tumour Volume) Clinician or planner applies margins to allow for set up errors and movement of patient (PTV: Planning Treatment Volume) Clinician or planner draws around other organs in proximity to the tumour (organs at risk) Planner optimises beams and arrangement of wedges/MLCs to get optimal coverage of PTV (i.e. all the area inside PTV received as close to the prescribed dose as possible) and minimises dose to organs at risk Outlining Creating GTV and PTV Organs at Risk Creating the plan The plan Dose to organs at risk and tumour Dose Volume Histograms SCOPE 1 Trial Design Study of Chemoradiotherapy in Oesophageal Cancer plus of minus Erbitux Stage 1 Patients with oesophageal cancer chosen to receive definitive CRT Stage 2 CRT Randomise CRT + cetuximab Treatment failure rate Overall survival n=180 n=240 (total of 420) Primary Endpoint Stage 1 Treatment failure rate (endoscopic assessment, biopsy CT scan) Stage 2 Overall survival Secondary Endpoint Toxicity Feasibility Toxicity Quality Assurance - RT Quality of Life Health Economics A7256 SCOPE 1 Study of Chemotherapy in Oesophageal Cancer plus of minus Erbitux PTV Dose Coverage Target Dose Volume of PTV receiving 95% of dose >99% of PTV to get 95% of dose Minimum dose to PTV Minimum dose to PTV should be greater than 93% Maximum dose to PTV Should be less than107% Organ at Risk Maximum Dose Maximum percentage of the OAR to receive max dose Combined Lungs 20Gy 25% Heart 40Gy 30% Spinal cord PRV 40Gy No part Liver 30Gy 60% Right or Left Kidney 20Gy 25% (single kidney) A7256 Why implement a QA RT programme? • Share experience to help establish best practice improve • Ensure consistent approach across all centres with a pre-trial test case (e.g. clinical outlines, planning techniques) • Ensure protocol adherence with on-trial QA (e.g. use of contrast, position verification) accuracy • Ensure treatment accuracy (e.g. audit visit to verify RT plan delivery) Provide ongoing support to clinicians and planners for difficult cases • Regular review of the protocol to incorporate new concepts of RT support delivery • Site Visit • Equipment audit • Dosimetry check Pre-trial test case Educational • Assessment of outlines • Assessment of plan • Assessment of form completion • RT specific protocol with planning tips • CD ROM with example cases Questionnaires • Baseline • Staff • Trial specific Assessment of patient cases QA RT Process • 1st case from each consultant and 10% sample • Plan Assessment Form for all patients • Check data is readable in VODCA QA process – who does what Site WCTU Velindre (CI / MP) Completes pre-trial educational exercise and test case Trials office chase up test cases, patient PAFs and plan data Pre-trial MP checks test case plan, drafts report including feedback on possible areas of improvement/advice Completes Plan Assessment Form (pre and on-trial) TM performs system check for readability of data (pre- and on-trial) On- trial advice where requested by WCTU Plans patient and exports DICOM data (pre- and on-trial) Checks PAF and highlights any out of range values (ontrial) On- trial assessment of patient cases Sends data(ftp server / CD) to trials office Patient data where deviation appears on PAF, full plan is sent to MP for assessment (on-trial) On-trial investigates deviations System check for the readability of exported data Examples of GTV Consistency Variation Images exported from VODCA Pros and Cons Pros Ensures the quality of data Provides data on consistency of dose and treatment Assesses adherence to protocol Educational component ensures minimum standard of treatment planning Helps improve networking and relationship with sites Cons Pre-trial can take time complete and lengthen set up times May put centres off taking part Can be time consuming chasing up data Additional resources needed to evaluate plans Conclusions – SCOPE 1 60 test case outlines and 40 plans have been returned, which means that the SCOPE 1 protocol and RT guidance document has been read and followed in all these centres The data collected suggest this has been a good educational exercise and highlights that there are variations between centres which can be addressed to improve consistency between both consultants and centres. A comprehensive QA programme can be implemented within a clinical trials unit with good collaboration and medical physics support. Conclusions The RTTQA group should be involved in all NIHR trials involving Radiotherapy Including a RTQA programme into a clinical trial should: – provide consistency in RT treatment, – raise the standards and consistency of RT – give validity to the results Collaborative working between WCTU and the Cardiff RTTQA group has provided a set of standards to use for subsequent trials and has proved to be a successful model Thank You Any Questions?