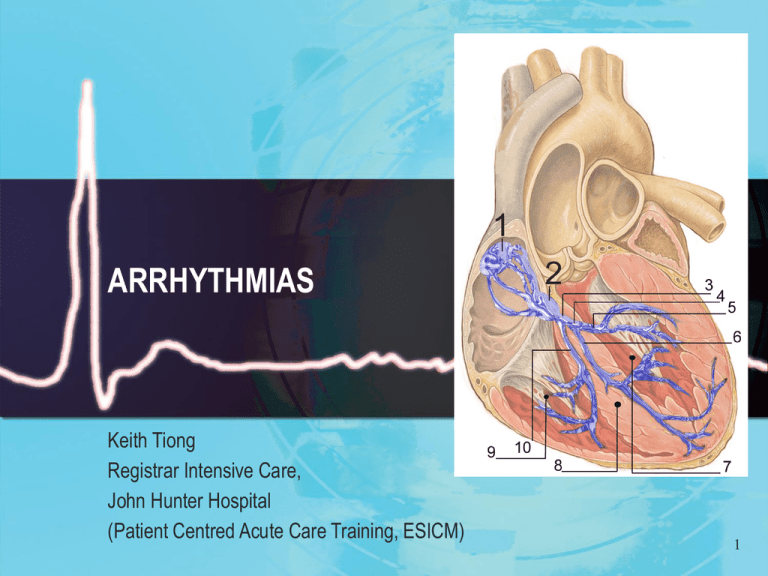
ARRHYTHMIAS
Keith Tiong
Registrar Intensive Care,
John Hunter Hospital
(Patient Centred Acute Care Training, ESICM)
1
College of Intensive Care Australia New Zealand
ELECTRICAL PROPERTIES OF THE HEART
● 1. General Instructional Objectives
● An understanding of the basis of electrical activity of cardiac muscle and its
relationship to basic mechanical events
● 2. Required Abilities
● a. To explain the ionic basis of spontaneous electrical activity of cardiac muscle cells
● (automaticity)
● b. To describe the normal and abnormal processes of cardiac excitation
● c. To explain the physiological basis of the electrocardiograph in normal and
common pathological states
● d. To describe the factors that may influence cardiac electrical activity
● e. To describe and explain the mechanical events of the cardiac cycle and correlate
this with physical, electrical and ionic events
2
College of Intensive Care Australia and New Zealand 2008
Basic Science Short Answer
● Question 7 - Outline normal impulse generation and conduction
in the heart. Describe the features present in a normal heart that
prevent generation and conduction of arrhythmias.
● Answer This question required description of the SA node, its primary
role and generation of the pacemaker potential and the influence of
the autonomic nervous system.
3
College of Intensive Care Australia and New Zealand 2008
Basic Science Short Answer
● A diagram of the conducting pathways, highlighting specialized tissues with fast or
slow conduction velocities would have been appropriate. The importance of the AV
node in preventing retrograde conduction and high rates conducted to the ventricles
(>220 / min) was often neglected in answers. A discussion of the Purkinje Fibres with
particular reference to the absolute and relative refractory periods was essential.
● Additional marks were awarded for mention of the atrial internodal pathways,
conduction within the ventricles from the endocardial to epicardial surfaces and the
significance of the compensatory pause in response to ectopic beats.
● Syllabus C1b 2.a, b;
● Reference: Cardiovascular Physiology, “Electrical Activity of the Heart” (Chapter 2),
Berne and Levy.
● 1 candidate (33%) passed this question.
4
‘An understanding of the basis of electrical activity of cardiac
muscle and its relationship to basic mechanical events’
●
●
●
●
●
●
●
●
●
●
●
●
Sinoatrial node (SAN)
Sited in the supepicardium, junction of right atrium (RA) and superior vena cava (SVC)
Extensive autonomic innervation
Abundant blood supply via SA nodal artery (proximal branch of RCA in 55% population) or left circumflex
coronary artery
Atrioventricular node (AVN)
Subendocardial structure within interatrial septum
Extensive autonomic innervation
Blood supply via AV nodal artery (distal branch of RCA, 90-95% population)
His bundle
Formed by Purkinje fibres emerging from distal AV node, forming tubular structure which runs through the
membranous septum to the muscular septum and divides into the bundle branches
Sparse autonomic innervation
Blood supply from AV nodal artery and septal branches of LAD artery
Patient-Centred Acute Care Training
European Society of Intensive Care
5
‘An understanding of the basis of electrical activity of cardiac
muscle and its relationship to basic mechanical events’
● Bundle Branches
● Anatomy varies
● Right bundle extends down right side of interventricular septum to base of anterior papillary muscle where
it divides
● Left bundle usually divides into two or three distinct fibre tracts - a left posterior and a left anterior
hemibundle
● Little autonomic innervation
● Extensive blood supply from RCA and LCA
● Normal conduction is initiated by the SA node, and results in a wave of depolarisation that spreads
through the atria, causing atrial contraction
● Atria and ventricles are electrically isolated from one another in all but one site - the AV node which serves
to:
— delay conduction between atria and ventricles, allowing time for the atrial component of ventricular
filling
— protect against the development of ventricular fibrillation (VF)
Patient-Centred Acute Care Training
European Society of Intensive Care
6
Managing the patient with rhythm disturbances
● Knowledge of the ionic currents responsible for the action
potential and the nature of cell-to-cell electrical transmission are
important for a comprehensive understanding of the cardiac
action potential and the interaction of drugs and hormones with
the ion channels
● Patient-Centred Acute Care Training
● European Society of Intensive Care
7
Conduction velocity and refractory periods
● Conduction Velocity
● Atrial/ventricular muscle fibers: 0.3-0.5 meters per second
● Specialized fibers for action potential propagation through the heart (e.g. Purkinje
fibers): 0.02-4 m per second
● Refractory Period
● Definition: amount of time following an action potential during which the normal
cardiac impulse cannot re-excite the previously excited tissue: this is the absolute
refractory period
— Duration -- normal absolute refractory period = 0.25-0.3 seconds
● Relative refractory period:
— Cardiac muscle may be excited, but with greater difficulty than normal.
— Duration: approximately 0.05 seconds (adds somewhat to the absolute refractory
period)
● Atrial refractory period (absolute refractory = 0.15 seconds; relative refractory = 0.03
seconds) -- shorter than ventricular refractory period. As a consequence, atrial
contraction rates may be significantly higher than ventricular contraction rates
8
Structure of ion channels
● Ion channels are proteins that traverse the plasma membrane. The major function of
ion channels is the rapid and selective movement of ions in and out the cell.
● The selective permeability of a channel for a particular ion in preference to others is
the basis for the classification of ion channels into Na+ , K+ , Ca++ channels among
others.
● The sodium current is primarily responsible for the depolarisation phase of the action
potential
● There are two major Ca++ currents in cardiac cells, the L-type and the T-type. L-type
currents (slow inward current).T-type current is faster and smaller than the L-type
current.
● Potassium currents. Several K+ currents are important in the cardiac tissue. Two key
currents are involved in the process of repolarisation (phase 3) during the action
potential and diastolic depolarisation (phase 4).
9
Structure of ion channels
● Phase 0:
— Activation of fast Na+ channel-- initial depolarization; slope &
— magnitude of a 0 will be dependent on the resting membrane
— potential (A in the diagram on the right)
● Phase 1:
— Partial repolarization; K+ efflux
● Phase 2:
— Ca2+ entry with continued K+ efflux = "plateau phase". Initial Ca2+ influx through slow L- type
Ca2+ channels initiates further Ca2+ release from and sarcoplasmic reticulum stores: Free
Ca2+ binds to contractile proteins (e.g. troponin C) promoting/enhancing muscle contraction
— catecholamines (sympathomimetic amines e.g. epinephrine, norepinephrine (Levophed)) increase
slow-inward Ca2+ currents-- a mechanism by which sympathomimetic agents enhance inotropism
● Phase 3
— This phase is dominated by K+ efflux, i.e. repolarization. The membrane potential moves towards
the original resting level. Phase 3 ccorresponds to the effective/absolute refractory period.
— Restoration of ionic gradients to "pre-action potential" levels requires the action of the Na+/K+
membrane ATPase-dependent transporter
● Phase 4
●
— This phase is between action potentials. In some cell types, phase 4 depolarization (diastolic
depolarization) can occur {especially, for example in "pacemaker" cells}.
10
SA nodal action potential characteristics/ Automaticity :
● "Slow-response" type, consistent with limited
●
●
●
●
●
●
●
●
●
●
fast-sodium channel activation involvement
second inward current carried by Ca2+, (ICa2+),
which is also depolarizing
and a third outward current carried by K+ (IK+),
the conductance of which tends to decrease during phase 4,
those leading to a net depolarizing effect.
Characteristic phase 4 depolarization (unstable
membrane potential drifting towards threshold–
phase 4 depolarization slope influenced by
sympathetic/parasympathetic stimulation as well as other factors.
11
College of Intensive Care Australia and New Zealand 2007
Basic Science Short Answer
● Q: Classify antiarrhythmic drugs, including their mechanisms of
action, and give an example of one drug from each group.
● A: This question again highlighted the importance of candidates
utilising a predetermined format or structure to their questions. Well
structured responses were less likely to overlook important details,
which was the predominate weakness for some candidates. A table
format was one useful way of displaying a good answer, for example -
12
College of Intensive Care Australia and New Zealand 2007
Basic Science Short Answer
13
● Quinidine: blocking the fast inward sodium current (INa). blocks the
slowly inactivating tetrodotoxin-sensitive Na current, the slow inward
calcium current (ICa), the rapid (IKr) and slow (IKs) components of the
delayed potassium rectifier current, the inward potassium rectifier
current (IKI), the ATP-sensitive potassium channel (IKATP) and Ito.
● Lignocaine: Block fast voltage gated sodium (Na+) channels
14
Electrogenic pumps
● In addition to the various ion channels, there are electrogenic
transporters which contribute to the membrane potential
● The Na+ /K+ pump: Adenosine triphosphatase (ATPase) dependent,
inhibited by digitalis glycosides, exchanges two potassium ions for
three sodium ions. The pump is electrogenic and increases the
intracellular negative potential. It promotes repolarisation and
maintains a low Na+ and high K+ inside the cell.
● Na+ /Ca++ exchanger: The Na+ /Ca++ exchanger extrudes three Na+
ions for each entering Ca++ ion when the membrane potential is more
positive than -40 mV, thereby increasing intracellular negativity
Patient-Centred Acute Care Training
European Society of Intensive Care
15
College of Intensive Care Australia New Zealand
ANTI-ARRHYTHMIC DRUGS
●
●
●
●
●
●
●
●
●
●
●
●
●
●
●
●
●
●
1. General Instructional Objectives
An understanding of the physiological and pharmacological basis of antiarrhythmic therapy
An understanding of the pharmacology of antiarrhythmic agents and their clinical
applications
2. Required Abilities
a. To classify antiarrhythmic agents by their electro-physiological activity and
mechanisms of action
b. To describe the pharmacology, with particular reference to the antiarrhythmic
properties, of:
· the sodium channel blocking agents (eg. lignocaine and flecainide)
· the beta blockers
· amiodarone, sotalol and ibutilide
· the calcium antagonists
· digoxin
· adenosine
· magnesium
c. To describe the adverse effects of the anti-arrhythmic agents with particular
reference to the potential pro-arrhythmic properties
16
College of Intensive Care Australia and New Zealand 2007
Basic Science Short Answer
● 5. Outline the pharmacology of amiodarone.
● Successful candidates applied, a systematic approach/format to
answer questions that refer tooutlining pharmacology of select drugs.
A number of useful mnemonics are suggested in the
● recommended texts for use when answering such a question. All
candidates correctly stated what amiodarone is used for but most
were not structured methodically and thus suffered from significant
omission.
17
College of Intensive Care Australia and New Zealand 2007
Basic Science Short Answer
● Amiodarone is an important class III anti-arrhythmic (with some
● characteristics of all 4 Vaughan-Williams classes). For a good pass candidates were expected
● to explain actions of amiodarone (eg blocks inactivated Na channels, decreases Ca current,
noncompetitive adrenergic blocking effect, blocks myocardial K channels which contributes to
● slowing of conduction and prolongation of refractory period in AV node, prolongs refractory
● period in all cardiac tissues, prolongs cardiac action potential duration) and it’s
● pharmacokinetics (eg bioavailability, large volume of distribution, high protein binding,
● complex metabolism and long elimination half life – 29 days)
● Syllabus: C2c
● Reference Text: Goodman and Gillman’s The Pharmacological basis of Therapeutics 11th ed
● 2006 and Pharmacology and Physiology in Anaesthetic Practice / Stoelting 4th ed 2006
18
Mechanisms of cardiac arrhythmias
● Abnormal automaticity and abnormal conduction are two major
causes of cardiac arrhythmias
● Automatic arrhythmias, such as automatic atrial tachycardia, require
no specific stimulus for initiation and may be persistent. Enhanced
phase 4 depolarisation would provoke such arrhythmias.
● Abnormal conduction may promote re-entry in heart muscle. Re-entry
is responsible for most clinically important arrhythmias including VT
associated with coronary artery disease, atrial flutter, AV nodal reentrant tachycardia, atrioventricular re-entry tachycardia as observed
in the Wolff-Parkinson-White Syndrome
● Patient-Centred Acute Care Training
● European Society of Intensive Care
19
Factors that increase the likelihood of arrhythmias are
commonly encountered in the intensive care setting:
● Pre-existing cardiac disease
● Treatment with anti-arrhythmics (this is with reference to the potential for
proarrhythmias e.g. class Ic)
● Recent macrovascular (i.e. occlusive coronary) event
● Microvascular disease causing ischaemia (e.g. diabetes mellitus, sepsis)
● Altered acid-base status
● High CO2
● Abnormal electrolyte balance
● Endogenous catecholamines (pain, anxiety)
● Exogenous catecholamines (inotropes)
● Presence of intracardiac catheters or pacing wires
● Suctioning, bronchoscopy, airway manipulation
● Deep anaesthesia (especially young patients)
● Anaesthetic drugs (e.g. pancuronium, methoxamine)
● Patient-Centred Acute Care Training
● European Society of Intensive Care
20
Management of arrhythmias in the critically ill is complex,
and for this reason we need some safe and simple rules.
● Rule 1. Not all arrhythmias need to be treated
● Rule 2. 'Electricity' is generally safer than drugs
● Rule 3. Correct all correctable abnormalities
● Rule 4. Treat all treatable ischaemia
● Rule 5. Consider your intravascular lines
● Rule 6. Consider drug toxicity
Patient
Centred Acute Care Training, ESICM
Patient-Centred Acute Care
Training
European Society of Intensive Care
21
Managing the patient with bradycardias
● 'Sinus node dysfunction' encompasses a heterogeneous group of
conditions, including:
● Sinus bradycardia
● Sinus arrest
● Sino-atrial block
● Sick sinus syndrome
● Sinus node dysfunction may be exacerbated by many medications,
but rarely needs treatment in the ICU setting.
● Patient-Centred Acute Care Training
● European Society of Intensive Care
22
'Sinus node dysfunction'
More Common
Sinus node fibrosis
Atherosclerosis of the SA artery
Congenital heart disease
Excessive vagal tone
Drugs
Less Common
Familial SSS (due to mutations in SCN5A)
Infiltrative diseases
Pericarditis
Lyme disease
Hypothyroidism
Rheumatic fever
23
Sinus node dysfunction in the context of acute myocardial
infarction
● This is a relatively common finding (5-30%) and is often associated
with concomitant AV nodal block. Usually no treatment is required,
unless in the case of cardiac failure, significant hypotension, or
continuing myocardial ischaemia.
● Intermittent sinus node dysfunction may respond to small doses of
atropine (note: rate response is unpredictable).
● If the bradycardia is prolonged, severe, aggravating ventricular
irritability, and not responding to atropine and isoprenaline then
temporary pacing may be indicated.
● Patient-Centred Acute Care Training
● European Society of Intensive Care
24
Atrioventricular (AV) conduction disease
● 1st degree AV block
● This refers to prolongation of the PR interval (>0.21 sec), and is strictly speaking not conduction block, merely
conduction delay. The QRS duration is normal (narrow QRS).
●
● 2nd degree AV block
●
● This results from intermittent failure of atrial depolarisation to reach the ventricles. Ventricular beats that do
occur result from normal conduction pathways..
● Type I (Mobitz I or Wenckebach)
● Progressive prolongation of the PR interval, then a 'dropped beat'
● Commonly occurs at the level of the AV node (narrow QRS)
● Type II (Mobitz II)
● Normal, constant PR interval, with intermittent 'dropped beats'
● Commonly occurs at the level of the AV node (narrow QRS)
● 3rd degree AV block (complete heart block)
●
● In complete heart block, although the atria depolarise normally, none of the atrial depolarisations reach the
ventricles, which beat independently in response to an infranodal pacemaker (wide QRS).
Patient-Centred Acute Care Training
European Society of Intensive Care
25
AV node dysfunction in the context of acute myocardial
infarction (MI)
● A degree of AV block occurs in 12-25% of patients with acute
myocardial infarction, most commonly in the context of inferoposterior
MI (with right ventricular involvement). AV block in this context usually
results from AV nodal ischaemia, is usually transient and usually
resolves. In anterior MI, AV nodal block usually occurs in the bundles
and can progress suddenly and without warning to complete AV block.
● Risk of progression to higher degrees of heart block/asystole, and
therefore requirement for temporary backup pacing varies.
Patient-Centred Acute Care Training
European Society of Intensive Care
26
Risk of progression to high-grade block
● Although 1 st degree and type I 2 nd degree block rarely require pacing(low risk of
progression), type I 2 nd degree block associated with a wide QRS (especially in the
context of anterior myocardial infarction) should have temporary backup pacing.
● Type II 2nd degree heart block (wide QRS), or type II 2nd degree heart block with
wide or narrow QRS complex in the context of anterior myocardial infarction should
have temporary backup pacing.
● Anterior MI with anything more than low-grade block may exhibit abrupt transition to
high-grade block with slow, unreliable ventricular escape rhythm. This combination is
associated with severe left ventricular dysfunction and high mortality.
● Patient-Centred Acute Care Training
● European Society of Intensive Care
27
Bundle branch block in the context of acute MI
● Development of BBB in anterior MI signifies a poorer prognosis
(due to large infarct size, left ventricular dysfunction and
conduction abnormalities). It is, however, difficult to predict
those patients who will need temporary pacing. Insertion of a
backup temporary pacing wire should be considered in the case
of
● 1st degree AV block + BBB
● New bifasicular block
● Alternating BBB
● Patient-Centred Acute Care Training
● European Society of Intensive Care
28
Cases for special consideration
● Infective endocarditis:
● Development of new AV block/BBB in a patient with infective
endocarditis implies an aortic root abscess (usually the non-coronary
cusp).
● All patients with aortic valve endocarditis should have daily 12-lead
ECGs performed specifically to look for conduction abnormalities
● Lyme disease:
● The commonest manifestation of the myocarditis of this condition is
AV block. This frequently resolves with antibiotic treatment, but may
require temporary pacing wire insertion.
Patient-Centred Acute Care Training
European Society of Intensive Care
29
Managing the patient with supraventricular tachycardias
● All supraventricular tachycardias may be caused and/or
exacerbated by inotropic agents. If possible, concomitant with
treating the arrhythmia, proarrhythmic drugs should be reduced.
● Patient-Centred Acute Care Training
● European Society of Intensive Care
30
Various clinical skills may be useful in the diagnosis of supraventricular
tachycardias, in addition to interpretation of the ECG
● Carotid sinus massage may increase AV block, and help in distinguishing some
tachycardias. Only perform if both carotid pulses are present and of equal strength
and there are no bruits. Perform gently to one side only but consider the risks in the
older patient or those with a history of transient ischaemic attacks or other
manifestations of cerebrovascular disease.
● Intravenous adenosine also increases AV block. This may help in diagnosis.
● Examination of the CVP line trace may be helpful in revealing the absence of an awave (for instance in AF), or the presence of cannon waves (in the case of av
dissociation).
● If the patient has temporary pacing wires inserted (either epicardially at time of
surgery, or transvenously as endocardial wires), simultaneous recordings can be
made from these to aid in diagnosis. For instance, the absence of P waves can
confirm atrial flutter or fibrillation in difficult cases: retrograde P waves - occurring
after the onset of each ventricular depolarisation - can be identified (via the atrial
ECG recording).
Patient-Centred Acute Care Training
European Society of Intensive Care
31
Paroxysmal SVTs
● Paroxysmal SVTs are divided into those arising from an
automatic focus and those resulting from re-entry. Of these, 810% result from increased automaticity, about 60% from AV nodal
re-entry, and 30% from AV junctional re-entry involving an
accessory pathway, often concealed. Junctional tachycardial
refers to accelerated junctional activity, and is uncommon except
with digoxin toxicity.
Patient-Centred Acute Care Training
European Society of Intensive Care
32
SVT
● The following are types of supraventricular tachycardias, each with a
different mechanism of impulse maintenance:
● SVTs from a sinoatrial source: Inappropriate sinus tachycardia,
sinoatrial reentrant tachycardia
● SVTs from an atrial source: Atrial tachycardia, flutter, fibrillation
● SVTs from an atrioventricular source (junctional tachycardia):
● AVRNT
● AV reentrant tachycardia (AVRT) - visible or concealed (including
Wolff-Parkinson-White syndrome)
33
Paroxysmal
atrial tachycardia
● Causes
● May derive from a number of general proarrhythmic factors in
ICU patients, or underlying structural heart disease. One of the
commonest causes is digoxin toxicity.
● Management
● Adenosine has been known to cardiovert some such patients.
● If tolerated, intravenous β -blockers are effective. Note, however,
that since chronic obstructive pulmonary disease is a common
cause of MAT, β -blockers may not be the best choice.
● In all cases, stop digoxin and treat toxicity if necessary.
Patient-Centred Acute Care Training
European Society of Intensive Care
34
Atrial flutter
● Causes
● In addition to the causes described above, specific additional causes to
remember include under/overfilling, and pulmonary embolism. Atrial flutter
may be resistant to chemical cardioversion.
● Management
● Digoxin is sometimes helpful in converting atrial flutter to atrial fibrillation,
which is easier to manage. Note, however, that the primary rationale for using
digoxin is to increase AV blockade.
● Overdrive atrial pacing may be used to cause cardioversion, if an atrial wire is
in use.
● Otherwise, management is similar to that of atrial fibrillation.
● Atrial flutter carries a risk of embolisation - anticoagulation may be advisable
before and after cardioversion (same guidelines as AF).
Patient-Centred Acute Care Training
European Society of Intensive Care
35
Atrial fibrillation
● Causes
● Specific causes to remember include under/overfilling, and
pulmonary embolism. Fever and sepsis should also be
considered in the ICU population.
● Treatment
● Therapeutic objectives in patients with atrial fibrillation in order
of importance are:
● Heart rate control
● Conversion to sinus rhythm
● Prevention of embolic complications
● Treatment of underlying (precipitating) cause
● Patient-Centred Acute Care Training
● European Society of Intensive Care
36
Atrial fibrillation
● Chemical cardioversion
● Clinical trials (but note, NOT in the ICU population) have
demonstrated increased success rate of transthoracic electrical
cardioversion for AF with ibutilide (class III potassium channel
blocker), but note the increased risk of torsade de pointes.
● Amiodarone (5 mg/kg slow 'push') may also result in cardioversion.
● In the perioperative state, magnesium-sulphate (34 mg/kg over 20
min, 0.1 mmol/kg) may be effective.
● Flecainide is contraindicated in patients with left ventricular
dysfunction or ischaemic heart disease. Up to 10% of patients
may develop acceleration of rate, or a proarrhythmic response.
● Patient-Centred Acute Care Training
● European Society of Intensive Care
37
Rate control
for atrial fibrillation
● To achieve rate control in atrial fibrillation acutely, digoxin has
the slowest onset of action and is not the drug of choice.
● Intravenous β -blockers or verapamil (0.075 mg/kg as a slow push)
provide rapid rate response, but are negatively inotropic.
● In the non-ICU population, digoxin together with atenolol has been
shown to be effective in controlling ventricular response rate in AF.
● Amiodarone is also rapidly effective in control of ventricular response
rate of AF in the ICU population.
● If ventricular response is uncontrolled, causing significant
haemodynamic compromise, and resistant to all conventional
manoeuvres, discussion with an electrophysiologist may be
helpful (with the potential for AV nodal ablation and insertion of a
permanent pacemaker).
Patient-Centred Acute Care Training
European Society of Intensive Care
38
Atrial fibrillation after cardiac and thoracic surgery
● Post-operative AF is a significant problem on the ICU, and many
trials have attempted to address this issue.
● Currently, the use of prophylactic drugs at the time of cardiac
surgery is not routine, however:
● Amiodarone (pre-operatively, 600 mg by mouth for 1 week prior to
cardiac surgery, and continued at 200 mg by mouth until discharge)
reduces the risk of AF.
● Amiodarone (intravenous immediately post-operatively and
continued for 48 hours) also reduces the risk of AF.
● Ibutilide successfully cardioverts patients with AF following cardiac
surgery.
● Patient-Centred Acute Care Training
● European Society of Intensive Care
39
AV nodal reentrant tachycardia
● These are usually based upon re-entry, two separate pathways within the AV
node having two different refractory periods and different conduction
velocities. These two pathways are connected proximally (close to the atrium)
and distally (close to the His bundle).
● Diagnosis
● Fast regular rhythm (classically rates of >150 bpm), paroxysmal, small QRS
(less than 0.12 sec). There will be no P waves preceding the QRS complex:
most often P waves are hidden within the QRS complex (common form),
although (retrogradely-conducted) negative P waves may sometimes be seen
following the QRS complex in leads (II, III, aVF) with a RP interval that is equal
to or longer than the PR interval (rare form).
● Treatment
● Carotid sinus massage or adenosine may both slow the rhythm, or cardiovert it.
● If the PSVT recurs, then verapamil is effective at terminating the rhythm and
preventing recurrence.
● Flecainide , β -blockers, and sotalol are also effective.
Patient-Centred Acute Care Training
European Society of Intensive Care
40
AVRT
● Orthodromic AVRT (More common) – Narrow complex tachycardia in which the wave
of depolarization travels down the AV node and retrograde up the accessory
pathway.
● Antidromic AVRT (Less common) – Wide complex tachycardia in which the wave of
depolarization travels down the accessory pathway and retrograde up the AV node.
41
Circus movement tachycardia (CMT)
Wolff-Parkinson-White (WPW) syndrome
● These are based upon the existence of an accessory AV connection
(Accessory Pathway, AP) between the atria and the ventricles. These pathways
not only lead to earlier activation of the ventricle following a supraventricular
impulse than during conduction over the AV node (so-called pre-excitation),
but also create the substrate for the re-entry circuit (CMT).
● Diagnosis
● Only patients with anterograde conduction have a delta wave on the
electrocardiogram. This ECG manifestation of pre-excitation is seen in
approximately 3/1000 ECGs. CMT may result in narrow or broad QRS
tachycardia.
● Orthodromic CMT: most often small QRS tachycardia unless pre-existing bundle
branch block, paroxysmal, regular rhythm, P waves are always separate from QRS:
usually RP<PR (fast conducting AP), RP>PR (slow conducting AP).
Patient-Centred Acute Care Training
European Society of Intensive Care
42
College of Intensive Care Australia and New Zealand 2009
SHORT ANSWER QUESTION PAPER 1
● Examine the ECG provided
a. List 3 abnormalities on this ECG
b. Name 2 drugs which are contraindicated in this
disorder
c. Name 2 complications of this disorder
43
College of Intensive Care Australia and New Zealand 2009
SHORT ANSWER QUESTION PAPER 1
●
●
●
●
●
●
●
●
●
●
●
●
●
a. List 3 abnormalities on this ECG
° Short PR
° Delta wave
° Wide QRS
° J wave (candidates mentioning this also received credit)
° Tall R wave in V1
b. Name 2 drugs which are contraindicated in this disorder
° Verapamil
° Digoxin
c. Name 2 complications of this disorder
° VF arrest
° Syncope
° AF/tachyarrhythmias
44
Managing the patient with ventricular tachycardias
● Ventricular extrasystoles
● In the context of the ICU, these should alert the physician to the
possibility of cardiac disease or irritability (mechanical or
chemical) of the heart. Appropriate management includes:
● Rigorous attention to correcting electrolyte imbalance
● Consider repositioning of any intracardiac lines
● In patients who have undergone cardiac surgery, or with underlying
ischaemic heart disease, potassium and magnesium should be
supplemented
Patient-Centred Acute Care Training
European Society of Intensive Care
45
Ventricular tachycardia (VT)
● Always consider the possibility of VT in a broad complex rhythm, even if the
heart rate is below 100bpm, especially if the patient is being, or has been
treated with anti-arrhythmic drugs, and also in the context of known or
suspected ischaemic heart disease.
● A broad complex tachycardia may be due to:
● Ventricular tachycardia
● Supraventricular tachycardia with aberrant conduction
● The likelihood of VT (vs SVT with aberrant conduction) increases if:
● Heart rate >170 bpm
● QRS duration >0.14 seconds
● The likelihood of SVT with aberrant conduction (vs VT) increases if the
morphology of the QRS complex on the 12-lead ECG is identical to that seen
prior to the onset of tachycardia.
Patient-Centred Acute Care Training
European Society of Intensive Care
46
Management
● Non-sustained VT
● Asymptomatic, normal left ventricular function - low risk of sudden death or serious
ventricular arrhythmias. Treat as for ventricular extrasystoles.
● Ischaemic heart disease with left ventricular ejection fraction <40% - high risk of
sudden death or serious ventricular arrhythmias. Address all treatable exacerbating
factors, seek cardiological opinion regarding catheterisation, possible intervention
(angioplasty or surgical referral), choice of anti-arrhythmic agent and consideration
for implantable cardioverter defibrillator (ICD).
● Recurrent non-sustained VT causing haemodynamic compromise. Address all
treatable exacerbating factors, consider lignocaine infusion, amiodarone infusion or
ventricular pacing (especially if VT emerges during period of relative bradycardia).
Patient-Centred Acute Care Training
European Society of Intensive Care
47
Newer interventions for the management of VT/VF
● Implantable cardioverter defibrillator (ICD)
● The development of smaller devices, with more sophisticated software, together with
increasing ease of implantation, and emerging evidence that ICDs improve survival
in certain patient groups is leading to increasing rates of implantation.
● ICDs:
● Implantable subcutaneously (pre-pectoral), with transvenous leads
● Able to diagnose ventricular tachycardia and ventricular fibrillation
● Able to deliver antitachycardia pacing and/or defibrillation
● Can be interrogated to determine number and length of arrhythmic episodes
● May be deactivated by placing a magnet directly over the generator site
● Do not preclude an operator delivering standard cardioversion/defibrillation
transcutaneously (take care not to place paddles over the device)
Patient-Centred Acute Care Training
European Society of Intensive Care
48
Newer interventions for the management of VT/VF
● Patients with improved survival with ICDs include:
● Reduced ejection fraction and inducible VT during electrophysiological
testing
● Survivors of arrests attributed to sustained VT with syncope, or
sustained VT and ejection fraction <40%
● Consider cardiological referral in such patients
● As increasing numbers of patients are fitted with these devices,
and those with ICDs are likely to come under the care of critical
care physicians at some stage during the course of their illness,
it is important that critical care physicians have some knowledge
of their potential functions and problems.
Patient-Centred Acute Care Training
European Society of Intensive Care
49
College of Intensive Care Australia and New Zealand 2010
SHORT ANSWER QUESTION PAPER 1
● Q: The following questions refer to implantable cardiac
pacemakers and
● implantable cardiac defibrillators.
● a) What is the effect of applying a magnet to these devices?
● b) What information can you gain from a chest X-Ray in a patient
with an
● implantable cardiac device?
● c) What are the advantages of DDD pacing compared to VVI
pacing?
● d) List 4 benefits of cardiac resynchronisation therapy.
50
College of Intensive Care Australia and New Zealand 2010
SHORT ANSWER QUESTION PAPER 1
●
●
●
●
●
●
●
●
●
●
●
●
●
Q: The following questions refer to implantable cardiac pacemakers and
implantable cardiac defibrillators.
a) What is the effect of applying a magnet to these devices?
ICD: it turns off antiarrhythmic programme but has no affect on backup
pacemaker
Pacemaker: It defaults to asynchronous mode or a fixed rate. Rate depends
on battery life.
b) What information can you gain from a chest X-Ray in a patient with an
implantable cardiac device?
• Single v dual chamber
• Biventricular or left ventricular (cardiac resynchronisation)
• Lead displacement or injury
• Number of devices present
51
College of Intensive Care Australia and New Zealand 2010
SHORT ANSWER QUESTION PAPER 1
●
●
●
●
●
●
●
●
●
●
●
●
●
c) What are the advantages of DDD pacing compared to VVI pacing?
• AV synchronisation maintained
• Avoids pacemaker syndrome
• Reduced incidence of AF
• Possible decreased thrombotic events
d) List 4 benefits of cardiac resynchronisation therapy.
• improved LVEF,CO and haemodynamics
• improved exercise tolerance
• decreased NYHA class
• decreased hospitilisation
• improved quality of life
Pass rate 6%
Highest mark 5.5
52
AV dyssynchrony syndrome ? Pacemaker syndrome
● Furman redefined pacemaker syndrome in a 1994 editorial in which
he included the following elements:
● Loss of AV synchrony
● Retrograde ventriculoatrial (VA) conduction
● Absence of rate response to physiologic need
53
College of Intensive Care Australia and New Zealand 2009
SHORT ANSWER QUESTION PAPER 1
● This ECG trace was taken from a 68 year old man, one hour
following aortic valve replacement for aortic stenosis. Atrial and
ventricular epicardial pacing wires are in place, and the pacing
mode is DDD.
● a) What problem is demonstrated?
● b) Outline the steps that you could take to address the problem.
54
College of Intensive Care Australia and New Zealand 2009
SHORT ANSWER QUESTION PAPER 1
●
●
●
●
●
●
●
●
●
●
●
●
a) What problem is demonstrated?
Intermittent failure of ventricular capture.
b) Outline the steps that you could take to address the problem.
Increase the ventricular output
Check the connections to the pacemaker and pacing connector leads
Reverse the polarity of the pacing to the ventricle
Replace pacemaker box and pacing connector leads
Unipolar pacing, with a cutaneous pacing stitch. This may fix the problem if one lead
is faulty.
Chronotropic therapy eg isoprenaline
Alternative pacing method: transcutaneous, transvenous
Open the chest and replace the epicardial wires
55
Consider referral for cardiological opinion in:
● Right ventricular outflow tract (RVOT) VT:
● Consider in a young patient with RVOT VT (LBBB, right axis) that
may terminate with adenosine , in the context of a structurally
normal heart.
● Idiopathic left ventricular tachycardia:
● Consider in VT with RBBB, left axis morphology that terminates
with verapamil , and a structurally normal heart.
● Bundle branch re-entrant VT:
● Consider in a patient with LBBB, syncope and dilated
cardiomyopathy
Patient-Centred Acute Care Training
European Society of Intensive Care
56
Torsade de pointes
● This ECG is a polymorphic ventricular tachycardia with a sinusoidal
electrocardiographic appearance due to the QRS complex undulating around the
baseline. The arrhythmia arises from prolonged myocardial repolarisation (seen on
the surface ECG as a prolonged QTc), which may be congenital or acquired. The
tachycardia is paroxysmal and may result in VF and sudden death
● Causes
● Electrolyte abnormalities especially hypomagnesaemia
● Anti-arrhythmic agents
● Hereditary long QT syndrome
● Bradyarrhythmias
● Myocardial ischaemia
● Neurological events
● Neuroleptics
● Antibiotics
● Toxins
57
Torsade de pointes
● Management
● Make the diagnosis and correct all exacerbating or causative
factors. Consider temporary pacing; intravenous magnesium;
ICD (although rarely necessary for torsades except in patients
with hereditary long QT).
58
ARRHYTHMIAS
●THE END
59
Pharmacologic Management of Atrial Fibrillation (AF):
● Prevention of thromboembolism
● Heart rate control versus rhythm control
● Optimizing the ventricular response
● Cardioversion of AF
● Maintenance of sinus rhythm
● Emerging therapies
60
Risk for Ischemic Stroke and Intracranial Bleeding as a
Function of Anticoagulation Intensity
Ischemic Stroke
Intracranial bleeding
20
Odds Ratio
15
10
5
1
1.0
2.0
3.0
4.0
5.0
6.0
International Normalized Ratio
Fuster V, et al. Circulation. 2006;114:e257-354.
61
7.0
8.0
Antithrombotic Therapy for Patients With AF
Risk Category
Recommended Therapy
No risk factors
One moderate-risk factor
Aspirin, 81 to 325 mg/d
Aspirin, 81-325 mg/d, or warfarin
(INR 2.0-3.0, target 2.5)
Warfarin (INR 2.0-3.0, target 2.5)*
Any high-risk factor or more than
1 moderate-risk factor
Less Validated or
Weaker Risk Factors
Female sex
Age 65-74 y
Coronary artery
disease
Thyrotoxicosis
Moderate-Risk Factors
High-Risk Factors
Age 75 y
Hypertension
Heart failure
LV ejection fraction ≤35%
Diabetes mellitus
Previous stroke, TIA,
or embolism
Mitral stenosis
Prosthetic heart
valve
*If patient has a mechanical valve, target INR is >2.5.
INR = international normalized ratio; LV = left ventricular; TIA = transient ischemic attack.
Fuster V, et al. Circulation. 2006;114:e257-354.
62
Heart Rate Control Versus Rhythm Control in
Persistent AF
RACE1
AFFIRM2
100
30
Rate control
25
Cumulative Mortality (%)
Event-free Survival (%)
90
80
Rhythm control
70
P = .08
60
50
0
20
Rhythm control
15
Rate control
10
5
0
0
6
12
18
24
30
36
0
1
Months
No. at Risk
Rate control
Rhythm control
256
266
239
243
232
224
222
218
4
3
2
5
Years
212
207
99
85
No. of Deaths
Rate control
Rhythm control
25
24
0
0
80 (4)
78 (4)
number (percent)
175 (9)
257 (13)
148 (7)
210 (11)
314 (18)
275 (16)
RACE = Rate Control Versus Electrical Cardioversion for Persistent AF; AFFIRM = AF Follow-up Investigation of Rhythm Management.
1. Van Gelder IC, et al. N Engl J Med. 2002;347:1834-1840.
2. Wyse DG, et al. N Engl J Med. 2002;347:1825-1833.
63
352 (24)
306 (21)
Rate Control Versus Rhythm Control:
Where We Are Now
● Rate control is a reasonable strategy in elderly patients with minimal
symptoms
● Deleterious effects of antiarrhythmic drugs may outweigh the benefits
of sinus rhythm
● There are no differences in quality of life, development of heart failure,
or thromboembolic events
● AF in the younger, more symptomatic patient was not addressed
● Effective strategies to maintain sinus rhythm with fewer side effects
are needed
64
Ventricular Rate Control in AF (As Defined in AFFIRM)
● Average heart rate at rest ≤80 beats/min and
● Either
— Maximum heart rate ≤110 beats/min during a 6-minute walk or
— Average heart rate ≤100 beats/min during 24-hour ambulatory Holter ECG
monitoring (at least 18 hours of interpretable monitoring) and no heart rate
>110% of the maximum predicted age-adjusted exercise heart rate
ECG = electrocardiography.
Olshansky B, et al. J Am Coll Cardiol. 2004;43:1201-1208.
65
Drug Therapy for HR Control in AF: Acute Management
Drug
Loading Dose
Onset
Maintenance Dose
Major Adverse Effects
5 min
5 min
60-200 μg/kg/min IV
NA
↓BP, HB, ↓HR, asthma, HF
↓BP, HB, ↓HR, asthma, HF
2-7 min
3-5 min
5-15 mg/h IV
NA
↓BP, HB, HF
↓BP, HB, HF
Days
0.5-1 mg/min IV
↓BP, HB, pulmonary toxicity, skin
discoloration, hypothyroidism,
hyperthyroidism, corneal deposits,
optic neuropathy, warfarin
interaction, sinus bradycardia
60 min
0.125-0.375 mg/d IV or po
Digitalis toxicity, HB, ↓HR
Patients without accessory pathway
Esmolol
Metoprolol
Diltiazem
Verapamil
500 μg/kg IV over 1 min
2.5-5 mg IV bolus over 2 min; up to
3 doses
0.25 mg/kg IV over 2 min
0.075-0.15 mg/kg IV over 2 min
Patients with accessory pathway
Amiodarone
150 mg over 10 min
Patients with heart failure and without accessory pathway
Digoxin
0.25 mg IV q2 h, to 1.5 mg
Amiodarone
Dosing, onset, and major adverse effects as above
BP = blood pressure; HB = heart block; HR = heart rate; HF = heart failure.
Fuster V, et al. Circulation. 2006;114:e257-354.
66
Drug Therapy for HR Control in AF: Long-term
Management
Drug
Loading Dose
Onset
Maintenance Dose
Major Adverse Effects
Metoprolol
Propranolol
Same as maintenance dose
Same as maintenance dose
4-6 h
60-90 min
↓BP, HB, ↓HR, asthma, HF
↓BP, HB, ↓HR, asthma, HF
Diltiazem
Same as maintenance dose
2-4 h
Verapamil
Same as maintenance dose
1-2 h
25-100 mg bid, po
80-240 mg/d in
divided doses, po
120-360 mg/d in
divided doses, po
120-360 mg/d in
divided doses, po
Heart rate control
↓BP, HB, HF
↓BP, HB, HF, digoxin interaction
Heart rate control in patients with heart failure and without accessory pathway
Digoxin
Amiodarone
0.5 mg/d po
800 mg/d for 1 wk, po
600 mg/d for 1 wk, po
400 mg/d for 4-6 wk, po
2 days
1-3 wk
0.125 to 0.375 mg/d po
200 mg/d po
Fuster V, et al. Circulation. 2006;114:e257-354.
67
Digitalis toxicity, HB, ↓HR
↓BP, HB, pulmonary toxicity, skin
discoloration, hypothyroidism,
hyperthyroidism, corneal
deposits, optic neuropathy,
warfarin interaction, sinus
bradycardia
Ventricular Rate Control in AF: Additional Caveats
● Digoxin is useful for patients with CHF or LV dysfunction and for
sedentary individuals
● Digoxin can be combined with -blockers or calcium channel blockers
to minimize bradycardia
● Amiodarone can be useful to control ventricular response (but
consider adverse effects)
● IV procainamide or ibutilide is useful to slow the ventricular response
in patients with preexcited AF (digoxin and AV-nodal blockers are
contraindicated)
● Permanent pacing may be necessary for bradycardia
CHF = congestive heart failure; AV = atrioventricular.
68
Patients Without a Change of Therapy (%)
Long-term Therapy With Rate Control Drugs: Efficacy of
-Blockers
100
80
60
Log rank = 77.02
P<.0001
40
-blocker
20
Calcium channel blocker
Digoxin alone
0
0
1
2
3
4
5
6
Time (Years)
BB:
CCB:
Digoxin:
N, Events (%)
777, 0 (100)
631, 0 (100)
315, 0 (100)
598, 147 (81)
461, 139 (77)
190, 104 (66)
500, 191 (75)
379, 187 (69)
142, 140 (53)
Olshansky B, et al. J Am Coll Cardiol. 2004;43:1201-1208.
69
315, 210 (71)
246, 220 (62)
92, 160 (45)
164, 213 (70)
128, 238 (56)
43, 165 (42)
35, 216 (68)
20, 247 (48)
5, 172 (29)
Rhythm Control: Pharmacologic Conversion of AF
(Duration of ≤7 Days)
Drug
Route of Administration
Agents with proven efficacy
Dofetilide
Flecainide
Ibutilide
Propafenone
Amiodarone
Oral
Oral or intravenous
Intravenous
Oral or intravenous
Oral or intravenous
Less effective or incompletely studied agents
Disopyramide
Procainamide
Quinidine
Should not be administered
Digoxin
Sotalol
Intravenous
Intravenous
Oral
Oral or intravenous
Oral or intravenous
Fuster V, et al. Circulation. 2006;114:e257-354.
70
Outpatient Therapy for Recent-Onset AF:
“Pill-in-the-Pocket” Approach
● Single-dose self-administration of propafenone or flecainide for AF of
<48 hours in duration
● Excluded: structural heart disease, sinus/AV-nodal dysfunction, QRS
>120 ms, ventricular rhythm <70 bpm, Brugada syndrome, systolic BP
<100 mm Hg
● Initial treatment given in hospital with monitoring
● Therapy was successful in 534 episodes (94%)
● Emergency room visits significantly reduced
● Pretreatment with -blocker or calcium channel blocker usually
required
Alboni P, et al. N Engl J Med. 2004;351:2384-2391.
71
Pharmacologic Therapy to Maintain Sinus Rhythm: Typical
Dosages and Adverse Effects
Drug
Daily Dose
Amiodarone*
100-400 mg
Disopyramide
400-750 mg
Dofetilide†
Flecainide
500-1000 μg
200-300 mg
Propafenone
450-900 mg
Sotalol†
160-320 mg
Potential Adverse Effects
Photosensitivity, pulmonary toxicity, polyneuropathy, GI
upset, bradycardia, torsades de pointes (rare), hepatic
toxicity, thyroid dysfunction, eye complications
Torsades de pointes, HF, glaucoma, urinary retention,
dry mouth
Torsades de pointes
VT, HF, conversion to atrial flutter with rapid conduction
through the AV node
VT, HF, conversion to atrial flutter with rapid conduction
through the AV node
Torsades de pointes, HF, bradycardia, exacerbation of
chronic obstructive or bronchospastic lung disease
*A loading dose of 600 mg/d is usually given for 1 month or 1000 mg/d for 1 week.
†Dose should be adjusted for renal function and QT-interval response during in-hospital initiation phase.
GI = gastrointestinal; VT = ventricular tachycardia.
Fuster V, et al. Circulation. 2006;114:e257-354.
72
Maintaining Sinus Rhythm: An Algorithm Based on
Underlying Heart Disease
Maintenance of Sinus Rhythm
No (or minimal)
heart disease
Hypertension
Coronary artery
disease
Heart failure
Flecainide
Propafenone
Sotalol
Substantial LVH
Dofetilide
Sotalol
Amiodarone
Dofetilide
Amiodarone
Dofetilide
Catheter
ablation
No
Yes
Flecainide
Propafenone
Sotalol
Amiodarone
Amiodarone
Dofetilide
Catheter
ablation
Catheter
ablation
LVH = left ventricular hypertrophy.
Fuster V, et al. Circulation. 2006;114:e257-354.
73
Amiodarone
Catheter
ablation
Catheter
ablation
Antiarrhythmic Drug Proarrhythmia: an Extension of
Pharmacologic Effects
Class IC toxicity:
Atrial flutter with 1:1 AV conduction
74
Class IA/III toxicity:
Torsades de pointes
Proarrhythmia With Antiarrhythmic Drugs
● Ventricular
— Torsades de pointes (class IA, III)
— Sustained monomorphic VT (class IC)
— Sudden death in coronary disease (class IC)
● Atrial
— Increased arrhythmias
— Conversion to atrial flutter (usually class IC)
● Abnormal conduction/impulse formation
— Increased ventricular rate during AF (class IA, IC)
— Sinus/AV-nodal dysfunction (nearly all drugs)
● Altered defibrillation thresholds (class I)
75
Risk Factors for Ventricular Proarrhythmia
VW Types IA and III Agents
VW Type IC Agents
Long QT interval (QTc 460 ms)
Long QT interval syndrome
Structural heart disease, substantial LVH
Depressed LV function*
Hypokalemia/hypomagnesemia*
Female sex
Renal dysfunction*
Bradycardia*
1. (Drug-induced) sinus node disease or AV block
2. (Drug-induced) conversion of AF to sinus rhythm
3. Ectopy producing short-long R-R sequences
Wide QRS duration (more than 120 ms)
Concomitant VT
Structural heart disease
Depressed LV function
*Some of these factors may develop later after the initiation of drug treatment.
VW = Vaughan-Williams.
Fuster V, et al. Circulation. 2006;114:e257-354.
76
Rapid ventricular response rate
1. During exercise
2. During rapid AV conduction
Risk Factors for Ventricular Proarrhythmia (Cont'd)
VW Types IA and III Agents
VW Type IC Agents
Rapid dose increase
High dose (sotalol, dofiletide), drug accumulation*
Addition of drugs*
1. Diuretics
2. Other QT-prolonging antiarrhythmic drugs
3. Nonantiarrhythmic drugs listed in
http://www.torsades.org
Previous proarrhythmia
After initiation of drug
Excessive QT lengthening
Rapid dose increase
High dose, drug accumulation
Addition of drugs
1. Negative inotropic drugs
*Some of these factors may develop later after the initiation of drug treatment.
Fuster V, et al. Circulation. 2006;114:e257-354.
77
Excessive (150%) QRS widening
On the Horizon
● Prevention of arrhythmogenic remodeling (structural
remodeling/fibrosis, inflammation, oxidative stress, atrial tachycardia
remodeling):
—
—
—
—
ACE inhibitors/ARBs/aldosterone antagonists
Statins
Omega-3 polyunsaturated fatty acids (fish oil)
Anti-inflammatory agents
● Atrial-selective agents
● Modifiers of gap junction coupling
● 5-Hydroxytryptamine 4 receptor antagonists
ACE = angiotensin-converting enzyme; ARB = angiotensin-receptor blocker.
78
Inhibition of Angiotensin II Signaling to Prevent AF:
a Meta-analysis
Study
Treatment,
n/N
Control,
n/N
Heart Failure
Ven Den Berg
SOLVD
VaHeFT
CHARM
Subtotal (95% CI)
2/7
10/186
116/2209
179/2769
307/5171
7/11
45/188
173/2200
216/2749
441/5148
RR (95% CI)
Weight,
%
RR (95% CI)
1.7
4.8
11.8
12.5
0.45 (0.13-1.57)
0.22 (0.12-0.43)
0.67 (0.53-0.84)
0.82 (0.37-0.85)
30.9
0.56 (0.37-0.85)
11.4
12.6
13.0
37.1
0.87 (0.68-1.11)
0.71 (0.59-0.85)
1.12 (0.95-1.32)
0.88 (0.68-1.19)
Test for heterogeneity chi-square = 15.01 df = 3 P = .0018
Test for overall effect z = 2.72 P = .007
Hypertension
CAPP
LIFE
STOPH2
Subtotal (95% CI)
117/5492
179/4417
200/2205
496/12,114
Test for heterogeneity chi-square = 13.34 df = 3 P = .0013
Test for overall effect z = 0.82 P = .4
RR = relative risk; CI = confidence interval.
Healey JS, et al. Am Coll Cardiol. 2005;45:1832-1839.
.1
.2
Favors treatment
79
1
5
Favors control
Inhibition of Angiotensin II Signaling to Prevent AF:
a Meta-analysis (Cont'd)
Study
Treatment,
n/N
Control,
n/N
9/79
18/70
27/149
22/75
32/75
54/150
RR (95% CI)
Weight,
%
RR (95% CI)
4.3
7.0
11.4
0.39 (0.19-0.79)
0.60 (0.37-0.97)
0.52 (0.35-0.79)
6.6
14.0
20.7
0.52 (0.31-0.87)
0.92 (0.83-1.02)
0.73 (0.43-1.26)
100.0
0.72 (0.60-0.85)
AF
Madrid
Ueng
Subtotal
Test for heterogeneity chi-square = 1.03 df = 1 P = .31
Test for overall effect z = 3.13 P = .002
Post-MI
TRACE
GISSI
Subtotal
22.790
665/8865
6897/9655
42/787
721/8846
763/9633
Test for heterogeneity chi-square = 13.34 df = 3 P = .0013
Test for overall effect z = 0.82 P = .4
Total
1517/27,089
2002/29,220
Test for heterogeneity chi-square = 48.50 df = 10 P = .00001
Test for overall effect z = 3.74 P = .0002
.1
.2
1
5
Favors treatment Favors control
Healey JS, et al. Am Coll Cardiol. 2005;45:1832-1839.
80
Use of Statins and AF in Patients With
Coronary Artery Disease
Probability of AF-free Survival
120%
100%
80%
60%
40%
Nonusers
Statin Users
20%
0%
0
1
2
3
4
5
Follow-up Time (Years)
Young-Zu Y, et al. Am J Cardiol. 2003;92:1379-1383.
81
6
7
8