the slides - ARV
advertisement
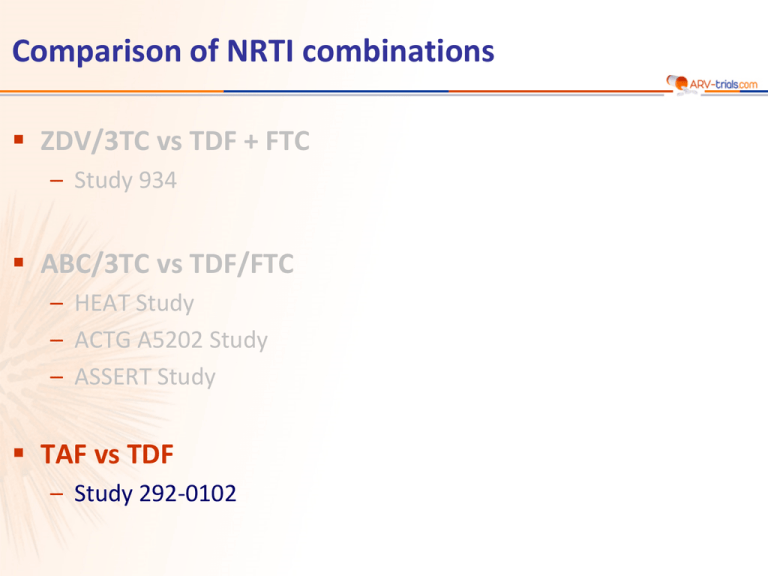
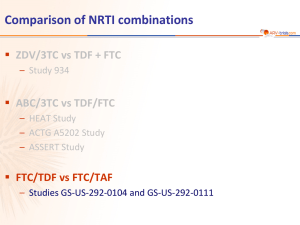
Comparison of NRTI combinations ZDV/3TC vs TDF + FTC – Study 934 ABC/3TC vs TDF/FTC – HEAT Study – ACTG A5202 Study – ASSERT Study TAF vs TDF – Study 292-0102 Study 292-0102: EVG/c/FTC/TAF QD vs EVG/c/FTC/TDF QD (Phase 2) Design Randomisation* 2:1 Double-blind W24 W48 EVG/c/FTC/TAF 150/150/200/10 mg QD N = 112 STR > 18 years ARV-naïve HIV RNA > 5,000 c/mL CD4 cell count > 50/mm3 eGFR > 70 mL/min EVG/c/FTC/TDF placebo N =58 EVG/c/FTC/TDF 150/150/200/300 mg QD STR EVG/c/FTC/TAF placebo *Randomisation was stratified by HIV RNA (< or > 100,000 c/mL) at screening Primary objective – Determine virologic efficacy of EVG/c/FTC/TAF – 150 patients provide 76% power to detect a 1.5% (SD of 3.3%) difference in hip bone mineral density in the EVG/c/FTC/TAF arm relative to the EVG/c/FTC/TDF arm 2920102/TAF phase 2 Sax PE. JAIDS 2014;67:52-8 Study 292-0102: EVG/c/FTC/TAF QD vs EVG/c/FTC/TDF QD (Phase 2) Baseline characteristics – Median viral load at baseline : 4.6 log10 c/mL (21% > 100,000 c/mL), median CD4+ cell count : 391 cells/mm3 (15% > 200/mm3) Main results – Discontinuation due to adverse events by W48 : 4 in TAF arm vs 0 in TDF arm – HIV RNA < 50 c/mL at W24 : 87.5% E/c/F/TAF vs 89.7% E/c/F/TDF – 3 patients in each arm met criteria for resistance testing (virologic failure [2 consecutive HIV RNA > 50 c/mL] with HIV RNA > 400 c/mL). Genotype was performed on confirmatory sample • E/c/F/TAF : no resistance detected • E/c/F/TDF : 2 patients developed resistance, 1 to NRTI, 1 to INSTI + NRTI – PK substudy : plasma TFV exposure was 91% lower with E/c/F/TAF than with E/c/F/TDF, as measured by AUCtau. Conversely, intracellular TFV-DP levels in PBMCs were 5.3-fold higher with E/c/F/TAF 2920102/TAF phase 2 Sax PE. JAIDS 2014;67:52-8 Study 292-0102: EVG/c/FTC/TAF QD vs EVG/c/FTC/TDF QD (Phase 2) Safety – Significantly less change in the E/c/F/TAF arm in BMD at hip (-0.62% vs -2.39%, p < 0.001) and lumbar spine (-1.0% vs -3.37%, p < 0.001) at W48, which were also significant at week 24 – In the E/c/F/TAF arm, 32% of patients had no decrease in hip BMD vs 7% in the E/c/F/TDF arm (p < 0.001) – Median change in eGFR by Cockcroft–Gault = -5.5 mL/min for E/c/F/TAF vs -10.1 mL/min for E/c/F/TDF (p = 0.041) – Renal tubular proteinuria [urine retinol-binding protein/creatinine ratio and urine b-2 microglobulin/creatinine ratio] was significantly lower in patients who received E/c/F/TAF : no cases of proximal tubulopathy – Grade 3-4 adverse events : 9.8% TAF vs 5.2% TDF – Most common treatment-emergent adverse events : nausea (21% vs 12%), diarrhea (16% in each arm) – Higher elevations in lipids with TAF 2920102/TAF phase 2 Sax PE. JAIDS 2014;67:52-8 Study 292-0102: EVG/c/FTC/TAF QD vs EVG/c/FTC/TDF QD (Phase 2) Grade 3 or 4 laboratory abnormalities E/C/F/TAF E/C/F/TDF Any 25% 17% LDL-cholesterol 9% 3% Creatine phosphokinase 6% 3% Neutropenia 5% 2% Amylase 3% 3% Urine red blood cells 2% 0 Total cholesterol 2% 0 1% / 1% 2% / 0 Gamma glutamyl transferase 1% 2% White blood cells 1% 0 Hypophosphatemia 1% 0 Urine protein 1% 0 Glucose 1% 2% Triglycerides 1% 2% ALT / AST 2920102/TAF phase 2 Sax PE. JAIDS 2014;67:52-8 Study 292-0102: EVG/c/FTC/TAF QD vs EVG/c/FTC/TDF QD (Phase 2) Summary of week 48 results – In this phase 2, randomised clinical trial, HIV-positive treatment-naive adults received STRs of E/c/F/TAF or E/c/F/TDF. Both STRs demonstrated high and comparable rates of virologic suppression through 48 weeks of therapy – Both regimens were well tolerated, with few discontinuations due to adverse events. Nausea occurred more frequently with E/c/F/TAF – Plasma concentrations of TFV were substantially (91%) lower with E/c/F/TAF than with E/c/ F/TDF, and the TAF regimen delivered 5.3 times the intracellular, physiologically active metabolite, TFV-DP, to PBMCs, which could translate into less end-organ toxicity and/or improved virologic control – Significant smaller decreases in bone mineral density through 48 with E/c/F/TAF than with E/c/F/TDF – Urinary RBP/creatinine and b-2 microglobulin/creatinine ratios were significantly lower in the E/c/F/TAF arm, which suggests that TAF has a lesser effect than TDF on the proximal renal tubular cell 2920102/TAF phase 2 Sax PE. JAIDS 2014;67:52-8