Cephalosporins - Changing resistance
advertisement

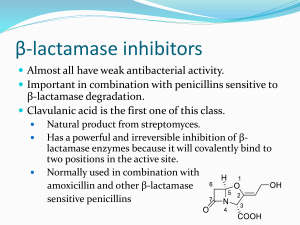
Dr.T.V.Rao MD Dr.T.V.Rao MD 1 Introduction to Cephalosporins.. Cephalosporins were first isolated from cultures of Cephalosporium acremonium from a sewer in 1948 by Italian scientist, Giuseppe Brotzu The first agent cephalothin (cefalotin) was launched by Eli Lilly in 1964 Dr.T.V.Rao MD 2 Cephalosporins …. B-Lactam antibiotics ( similar to penicillin's) Broad spectrum in action. Act by inhibition of cell wall synthesis Bactericidal Inactive against : enterococci, MRSA, legionella , mycoplasma, chlamydia spp. Widely used in surgical procedures to reduce the risk of post operative infections Dr.T.V.Rao MD 3 Antimicrobial activity of Cephalosporins The site of action of beta-lactam antibiotics is the penicillin binding proteins (PBPs) on the inner surface of the bacterial cell membrane that are involved in the synthesis of the cell wall Cephalosporins are bactericidal agents All bacterial cells have a cell wall that protects them. Cephalosporins disrupt the synthesis of the peptidoglycan layer of bacterial cell walls, which causes the walls to break down and eventually the bacteria die. Dr.T.V.Rao MD 4 Classification is based on spectrum of activity Cephalosporins are grouped into "generations" based on their spectrum of antimicrobial activity. The first cephalosporins were designated first generation while later, more extended spectrum cephalosporins were classified as second generation cephalosporins. So continued Generations Dr.T.V.Rao MD 5 Basis of Classification … Each newer generation of cephalosporins has significantly greater gram-negative antimicrobial properties than the preceding generation Fourth generation cephalosporins, however, have true broad spectrum activity Dr.T.V.Rao MD 6 st 1 generation Cephalosporins First generation cephalosporins are moderate spectrum agents Effective against gram +ve aerobes They are effective for treating staphylococcal and streptococcal infections and therefore are alternatives for skin and soft-tissue infections, as well as for streptococcal pharyngitis. Dr.T.V.Rao MD 7 The 1st generation cephalosporins are: Cefadroxil Cephalexin Cephaloridine Cephalothin Cephapirin Cefazolin Cephradine Dr.T.V.Rao MD 8 st 1 Generation Active against G+ cocci ( except. Enterococci & MRSA ): s.pneumoniae, s.pyogenes,s. aureus, S. epidermidis Indicated for streptococcal pharyngitis ( e.g. cephalexin) Commonly used ( eg. Cefazolin) as prophylactic for surgical procedures. Modest activity against G- bacteria Dr.T.V.Rao MD 9 nd 2 generation Cephalosporins Their antibacterial spectrum is broader than that of 1st generation cephalosporins and includes some gram -ve pathogens They are also more resistant to beta-lactamase They are useful agents for treating upper and lower respiratory tract infections and sinusitis Dr.T.V.Rao MD 10 nd 2 generation cont... These agents are also active against E. coli, Klebsiella and Proteus, which makes them potential alternatives for treating urinary tract infections caused by these organisms Dr.T.V.Rao MD 11 nd 2 Generation Cephalosporins .. Cefoxitin Cefuroxime Cefuroxime axetil Cefaclor Cefprozil Dr.T.V.Rao MD 12 rd 3 generation Cephalosporins They have an extended spectrum of action against gram -ve organisms Resistant to betalactamases Dr.T.V.Rao MD 13 rd 3 generation cont... The parenteral third generation cephalosporins (ceftriaxone and cefotaxime) have excellent activity against most strains of Streptococcus pneumoniae, including the vast majority of those with intermediate and high level resistance to penicillin Dr.T.V.Rao MD 14 Third Generation Cephalosporins Ceftriaxone Cefotaxime Ceftazidime Cefoperazone Cefixime Dr.T.V.Rao MD 15 THIRD GENERATION They have enhanced G- activity, H. influenza, N. meningitidis, N.gonorrhea, P. aeruginosae, M. catarrhalis, E.coli, most Klebsiella Ceftriaxone has long half-life . Not advised in neonates (interferes with bilirubin metabolism ) Cefotaxime preferred in neonate ( does not interfere with bilirubin metabolism ), as may ceftriaxone. Ceftazidime & cefoperazone have excellent activity against P.aeruginosa. Cefixime has similar activity to amoxicillin & Cefaclor for actute otitis media Dr.T.V.Rao MD 16 th 4 generation cephalosporins 4th generation cephalosporins are extended spectrum agents with similar activity against gram-positive organisms as first generation cephalosporins. They also have a greater resistance to beta-lactamases than the third generation cephalosporins. Many can cross blood brain barrier and are effective in meningitis. Dr.T.V.Rao MD 17 th 4 Generation Cephalosporins... Cefepime Cefluprenam Cefozopran Cefpirome Cefquinome Dr.T.V.Rao MD 18 Fourth Generation Cefipime Active against G+ bacteria > than Cefazolin against s. pyogenes, S.pneumoniae but lower against s. aureus. Similar to cefotaxime against E.coli & K. pneumoniae but < for p. aeruginosa. Dr.T.V.Rao MD 19 Pharmacokinetic consideration They are organic acids and are hydrophilic They generally have poor oral bioavailability as they unstable in acid environments They are readily excreted by the kidneys, via tubular secretion in the proximal convoluted tubule. This results in high concentrations of the drug in urine. Exceptions are: Cephalexin which is stable in acid and so suitable for oral dosing. Cefoperazone is excreted in bile rather than in urine. Dr.T.V.Rao MD 20 Why Cephalosporins are Widely Prescribed Antibiotics Broad spectrum of activity Stability to -lactamase Oral and parenteral preparations Widely accepted Treats ‘day to day’ as well as ‘serious infections’ High safety profile Dr.T.V.Rao MD 21 Cephalosporins Emerging resistance patterns -Limitations III & IV generation cephalosporins were available only as parenteral formulations Pharmacoeconomics Dr.T.V.Rao MD 22 Why detect ESBL producers? may: ESBL producers • Appear Sensitive to some cephalosporins in vitro • Show major inoculum effects • Fail in therapy, despite appearing susceptible Dr.T.V.Rao MD 23 Detection Strategy: step 1 Screen Enterobacteriaceae with : • Cefpodoxime- best general ESBL substrate • Cefotaxime & ceftazidime- good substrates for CTX-M & TEM/SHV, respectively Spread of CTX-M into community means screening must be wider than before Dr.T.V.Rao MD Ref http://www.hpa.org.uk 24 Detection of ESBLs: step 2 Seek ceph/clav synergy in ceph R isolates Double disc Combina tion disc Etest Dr.T.V.Rao MD Ref http://www.hpa.org.uk 25 Double Disk Method Dr.T.V.Rao MD 26 Etest for ESBLs Cefotaxime Cefotaxime + Clavulanate Dr.T.V.Rao MD 27 Pitfalls in ESBL detection • Methods optimised for E. coli & Klebsiella • More difficult with Enterobacter – clavulanate induces AmpC; hides ESBL • Best advice is to do synergy test (NOT SCREEN) with 4th gen cephalosporins Dr.T.V.Rao MD 28 Synergy tests with 4-gen cephalosporins Cefepime/clav (Mast & AB Biodisk) Cefpirome clav (Oxoid) Devt. driven by spread of clonal E. aerogenes with TEM-24 in Belgium & France Sensitivity for weak ESBLs remains to be proven Cefpirome & cefepime products need comparison Dr.T.V.Rao MD 29 Bacteria not to test for ESBLs Acinetobacter –Often S to clavulanate alone S. maltophilia –+ve result by inhibition of L-2 chromosomal -lactamase, ubiquitous in the species Dr.T.V.Rao MD 30 Role of CLSI in Revising Breakpoints in Antibiotic Resistance Briefly, revising breakpoints involves systematic review of microbiological, pharmacologic, and clinical data. Recognized experts, sponsors (pharmaceutical industry), and regulators participate in the process which includes discussions at public meetings of the CLSI Subcommittee on Antimicrobial Susceptibility Testing that take place twice a year. When establishing original breakpoints for new agents, controlled clinical trial data are required Dr.T.V.Rao MD 31 Follow the New Guidelines CLSI 2010 Guidelines for cephalospins for Enterobacteriaceae in accordance with the 2010 Clinical Laboratory Standards Institute (CLSI) recommendations. The following changes will be made to comply with the CLSI. Dr.T.V.Rao MD 32 Why do breakpoints sometimes need to be revised? Breakpoints need to be revised due to changing resistance mechanisms and bacterial population distributions, changing science leading to a better understanding of the pharmacologic determinants of clinical response, and adoption of “best practices” by clinicians. Dr.T.V.Rao MD 33 Enterobacteriaceae Rapid Spread of resistance The rapid and disturbing spread of: extended-spectrum ßlactamases AmpC enzymes carbapenem resistance metallo-β-lactamases KPC and OXA-48 βlactamases quinolone resistance Dr.T.V.Rao MD 34 What breakpoints were revised in 2010? Select cephalosporin and aztreonam breakpoints for Enterobacteriaceae were revised as noted below (for comparison, the old breakpoints are included): Dr.T.V.Rao MD 35 Extended-Spectrum βLactamases β-lactamases capable of conferring bacterial resistance to the penicillin's first-, second-, and third-generation cephalosporins aztreonam (but not the cephamycins or carbapenems) These enzymes are derived from group 2b βlactamases (TEM-1, TEM-2, and SHV-1) differ from their progenitors by as few as one AA Dr.T.V.Rao MD 36 CTCTX-M-type ESBLs X-M-type ESBLs Until 2000, most ESBL producers were hospital Klebsiella spp. with TEM and SHV mutant βlactamases Now, the dominant ESBLs across most of Europe and Asia are CTX-M enzymes, which originated as genetic escapes from Kluyvera spp Currently recognized as the most widespread and threatening mechanism of antibiotic resistance, both in clinical and community settings 80% of ESBL-positive E. coli from bacteraemias in the UK and Ireland are resistant to fluoroquinolones 40% are resistant to gentamicin Dr.T.V.Rao MD Livermore, DM J. Antimicrob. Chemother 2009 37 Enterobacteriaceae: Revised Breakpoints for Cephalosporins CLSI 2009 Agent CLSI 2010 S I R S I R Cefazolin ≤8 16 ≥32 ≤1 2 ≥4 Cefotaxime ≤8 16-32 ≥64 ≤1 2 ≥4 Ceftriaxone ≤8 16-32 ≥64 ≤1 2 ≥4 Ceftazidime ≤8 16 ≥32 ≤4 8 ≥16 Aztreonam ≤8 16 ≥32 ≤4 8 ≥16 Cefipime ≤8 16 Dr.T.V.Rao MD ≥32 ≤8 16 ≥32 38 Disk diffusion breakpoints (mm): Agent Cefazolin Cefotaxime Ceftizoxime Ceftriaxone Ceftazidime Aztreonam Old (M100-S19) S ≥18 ≥23 ≥20 ≥21 ≥18 ≥22 I 15-17 15-22 15-19 14-20 15-17 16-21 R ≤14 ≤14 ≤14 ≤13 ≤14 ≤15 Revised (M100-S20) S I R NA NA NA ≥26 23-25 ≤22 ≥25 22-24 ≤21 ≥23 20-22 ≤19 ≥21 18-20 ≤17 ≥21 18-20 ≤17 S – susceptible I – Intermediate R – Resistant. Dr.T.V.Rao MD 39 Following MIC breakpoints were reevaluated for Enterobacteriaceae but were not revised Agent M100-S19 S Cefuroxime ≤8 Cefepime ≤8 Cefotetan ≤16 Cefoxitin ≤8 M100-S20 I R S I R 16 16 32 16 ≥32 ≥32 ≥64 ≥32 ≤8 ≤8 ≤16 ≤8 16 16 32 16 ≥32 ≥32 ≥64 ≥32 S – susceptible I – Intermediate R – Resistant Dr.T.V.Rao MD 40 Why were the breakpoints for cefepime and cefuroxime (parenteral) not revised? The cefepime breakpoints were not revised based upon clinical trial data and PK-PD evaluations. The clinical trial data showed cefepime efficacy for patients infected with isolates that tested cefepime susceptible (MIC ≤8 μg/ml), but produced an ESBL Dr.T.V.Rao MD 41 Why are there no disk diffusion breakpoints for Cefazolin? Studies have not yet been completed to identify the zone diameter breakpoints that correlate with the revised MIC breakpoints for Cefazolin. Initial studies did not reveal clear zone diameter breakpoints and disk diffusion testing of Cefazolin may require a new disk with alternate disk content. Dr.T.V.Rao MD 42 Cephalothin group Cephalothin is now classified under Test/Report Group U for Enterobacteriaceae. Results for cephalothin can be used to represent activities of several other oral FDA-approved agents for treatment of urinary tract infections which include cefadroxil, cefpodoxime, cephalexin, and loracarbef. Dr.T.V.Rao MD 43 Need for Changing Recommendations The ESBL testing recommendations were to be a short term solution to address a new mechanism of resistance. Subsequently, additional mechanisms of resistance have been identified (e.g., new types of ESBLs and AmpC-like enzymes) and with increased frequency multiple enzymes are identified in a single isolate which can complicate ESBL testing (1). These issues coupled with improved understanding of the PK-PD determinants of efficacy with cephalosporins and monobactams resulted in the decision to revise the breakpoints. Dr.T.V.Rao MD 44 Measuring the Revised Zones is Advantageous The revised breakpoints eliminate the need to perform ESBL screen and confirmatory tests for making treatment decisions. Phenotypic tests for ESBL detection and confirmation are less accurate when multiple enzymes are present (e.g., falsenegative results occur when isolates express both ESBLs and AmpC-type enzymes) (13) and the presence of multiple enzymes are more common in contemporary isolates (4, 8). The MIC of an isolate correlates better with clinical outcome than knowledge of resistance mechanisms (e.g., ESBLs) Dr.T.V.Rao MD 45 Follow me for More Articles of Interest on Infectious Diseases Dr.T.V.Rao MD 46 Programme Created by Dr.T.V.Rao MD for ‘e’ learning resources for Medical and Paramedical Students in Developing world Email doctortvrao@gmail.com Dr.T.V.Rao MD 47