icu nutrition

Nehad Mohammed Osman
Lecturer of chest diseases
Hippocrates 400 B.C.
Why do we feed ICU patients?
Which patients should we feed?
When should we start to feed them?
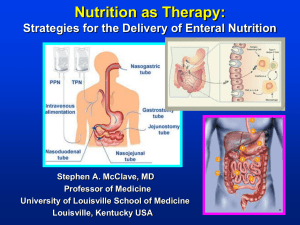
Which route should we feed by?
How much feed should we give?
What should the feed contain?
As many as 40% of adult patients are seriously malnourished when admitted to the hospital.
In addition, two-thirds of all patients' nutritional state deteriorates during their hospital stay. Acute illness further exacerbates patients' poor nutritional status by increasing their metabolic rate and impairing the allocation of nutritional substrates.
Historically, the intensive care unit population has been routinely malnourished. One explanation is that nutritional issues are often not initially explored because the traditional, more obvious issues involving cardiac, pulmonary, or neurological status are deemed as more critical.
Alterations in these body systems produce more immediate body responses, such as hypotension, chest pain, shortness of breath or unresponsiveness, and therefore typically garner prompt attention. In contrast, the debilitating effects of malnutrition may not be clinically apparent for several days. As such, preventable outcomes associated with malnourishment may not be noticed until patient complications experiences
CRITICAL COMPLICATIONS
Malnutrition in the critically ill, mechanically ventilated patient has an adverse effect on all physiological processes. It increases the risks for infection and pulmonary edema. Also, nutrition deficits decrease phosphorus needed to produce adenosine triphosphate for cellular energy, reduce ventilatory drive, and impair surfactant production. These malnourished patients are difficult to wean from the ventilator because of muscle fatigue caused by diaphragmatic and skeletal muscle weakness and/or reduced muscle endurance.
PHYSIOLOGIC EFFECTS OFMALNUTRITION
Pulmonary
Decreased diaphragmatic contractility
Depressed hypoxic drive & ventilatory drive to CO2
Cardiac
Decreased contractility/response to inotrope
Ventricular dilatation
Renal
Decreased GFR
Impaired Na+ excretion
Hepatic
Altered CHO, protein & fat metabolism • Decreased protein synthesis •
Decreased drug metabolism
Impaired bilirubin excretion
Hematology •
Anaemia & coagulopathy.
Depressed T-cell functions . Impaired chemotaxis and phagocytosis
GIT
Decreased gut motility
Gut atrophy
Increase gut permeability to intestinal bacteria
few data directly compare feeding with no feeding – two trials and one metaanalysis suggest worse outcomes in un(der)fed patients catabolism of critical illness causes malnutrition. malnutrition closely associated with poor outcomes many ICU patients are malnourished on admission
One nutrient, protein, is especially vital in critical illness. Decreased protein intake associated with malnourishment decreases serum albumin level, which leads to a decreased intravascular and intracapillary oncotic pressure. This decreased pressure causes fluid to leak from the intravascular space into the interstitial space, a condition referred to as third spacing, which causes edema.
Ultimately, malnutrition increases patient mortality and hospital cost by prolonging a patient's hospital stay.
Critically ill patients who receive prolonged mechanical ventilation without nutritional support have a 20% to 40% increased risk of developing ventilator-associated pneumonia
(VAP) compared to those who are fed enterally within 48 hours of intubation. VAP is associated with a 20% to 25% increased relative mortality and an increased hospital stay of at least four days.
Increased morbidity and mortality
Prolonged hospital stay
Impaired tissue function and wound healing
Defective reduced muscle function, respiratory and cardiac function
Immuno-suppression, increased risk of infection
CIPs lose around 2%/day muscle protein
treat existing malnutrition
minimise (but not prevent) the wasting of lean body mass that accompanies critical illness
important to identify existing malnutrition clinical evaluation is better than tests
History
1. Weight change (>5% of usual body weight in 3 weeks or >10% in
3 months).
2. Changes in food intake.
3. Gastrointestinal symptoms.
4. Functional impairment.
Physical Examination
1. Loss of subcutaneous fat – especially in chest and triceps (body mass index <20).
2. Muscle wasting – especially at temporal region, deltoids and gluteals.
3. Oedema.
4. Ascites.
which patients can safely be left to resume feeding themselves?
14 days’ starvation - dangerous depletion of lean body mass mortality rises in ICU patients with a second week of severe under-feeding
5 days without feed increases infections but not mortality one view is therefore that 5-7 days is the limit
however
ACCEPT study fed all patients not likely to eat within 24 hours
one meta-analysis suggests reduced infections if patients are fed within 48 hours
one meta-analysis of early TPN versus delayed EN found reduced mortality with early feeding
all malnourished patients
all patients who are unlikely to regain normal oral intake within either 2 or 5-7 days depending on your view
without undue delay once the patient is stable this will usually be within 48 hours of ICU admission
ACCEPT study aimed to start within 24 hours of
ICU admission
Studies suggest that initiating nutritional support within 24 to 48 hours of intubation helps maintain lean body mass and immune function, thereby improving clinical outcomes, lowering infection rates and reducing hospital length of stay.
Starting enteral nutrition near the beginning of an acute illness has several benefits. This type of nutrition improves immune function and augments the cellular antioxidant system. There is also a decrease in the body's hypermetabolic response to tissue injury. Other advantages include better nitrogen balance and improved wound healing. For this reason, it is imperative the nurse requests a nutrition consult within 24 to 48 hours following endotracheal intubation
enteral feeding is claimed to be superior because
it prevents gut mucosal atrophy
it reduces bacterial translocation and multi-organ failure
lipid contained in TPN appears to be immunosuppressive
EN preferred for majority on pragmatic grounds alone
TPN obviously necessary for some if there is serious doubt that EN can be established in 2 (or 5, 7…) days
commence TPN
maintain at least minimal EN
keep trying to establish EN
does EN reduce infections?
◦ pancreatitis - probably
◦ abdominal trauma - probably (2 trials of 3)
◦ head injury - evenly balanced
◦ other conditions – no clear conclusion
◦ Lipman reviewed 31 trials and found no consistent effect
◦ meta-analysis by Heyland et al found reduced infections
EN is definitely a risk factor for VAP
Decisions on route, content, and management of nutritional support are best made by multidisciplinary nutrition teams enteral feeding is
◦ cheaper
◦ easier
◦ and therefore preferable in most cases parenteral feeding is obviously necessary in some
ETF can be used in unconscious patients, those with swallowing disorders, and those with partial intestinal failure.
It may be appropriate in some cases of anorexia nervosa.
Early post pyloric ETF is generally safe and effective in postoperative patients, even if there is apparent ileus
Early ETF after major gastrointestinal surgery reduces infections and shortens length of stay
In all post surgical patients not tolerating oral intake, ETF should be considered within 1–2 days of surgery in the severely malnourished, 3–5 days of surgery in the moderately malnourished, and within seven days of surgery in the normally or over nourished.
If there are specific contraindications to ETF, parenteral feeding should be considered. If patients are taking .50% of estimated nutritional requirements, it may be appropriate to delay instigation of ETF.
Fine bore (5–8 French gauge) nasogastric (NG) tubes should be used for ETF unless there is a need for repeated gastric aspiration or administration of high viscosity feeds/drugs via the tube. Most fibre enriched feeds can be given via these fine bore tubes
Long term NG and NJ tubes should usually be changed every 4–6 weeks swapping them to the other nostril
Gastrostomy or jejunostomy feeding should be considered whenever patients are likely to require
ETF for more than 4–6 weeks and there is some evidence that these routes should be considered at 14 days
ENTERAL FEEDING RESIDUAL VOLUMES
Residual volumes are routinely checked as a way to assess tube-feeding tolerance and help to assess a patient's risk for aspiration. Although checking residual volume is a common clinical practice, there is no data correlating a specific residual volume with increased aspiration events. The single best measure a nurse can do to prevent aspiration of enteral feedings and therefore reduce the risk of VAP is to keep the patient's head of bed elevated at least 30 degrees.
The American Gastroenterological
Association recommends elevating the head of bed to a minimum of 30 to 45 degrees to reduce the risk of microaspiration.
Gastric residual volumes greater than 200 ml to 250 ml are generally considered high in critically ill patients with an artificial airway in place. It is interesting to note that the combined secretion of saliva and gastric fluids may total up to 188 ml/hr, which brings one to question whether a tube feeding residual of 250 ml is really an accurate reflection of poor absorption. It is not recommended to automatically stop a tube feeding for an isolated high gastric residual volume. A residual recheck should be done one hour before tube feedings are held. Though the gastric residual is a factor in aspiration, ongoing studies contend there is no consistent relationship between aspiration and gastric residual volumes. However, aspiration does occur significantly more often when volumes are high.
Type Complication
Insertion Nasal damage, intracranial insertion, pharyngeal/oesophageal pouch perforation, bronchial placement, variceal bleeding
PEG/PEJ insertions Bleeding, intestinal/colonic perforation
Post insertion trauma Discomfort, erosions, fistulae, and strictures
Displacement Tube falls out, bronchial administration of feed
Reflux Oesophagitis, aspiration
GI intolerance Nausea, bloating, pain, diarrhoea
Metabolic Refeeding syndrome, hyperglycaemia, fluid overload, electrolyte disturbance
PEG, percutaneous endoscopic gastrostomy; PEJ, percutaneous endoscopic jejunostomy; GI, gastrointestinal.
Complication
Vomiting
Abdominal Distension and/or cramping or tenderness (if detectabl
Severity Definition Treatment
(occurrence)
Mild
1-4+ times/12 hrs Place NG to suction, check function. Check existing NG function. ▼TF infusion rate by
50%; Notify primary team
Hx and/or physical evidence Check for constipation;
Maintain TF infusion rate;
Reexamine in 6 hrs if indicators remain mild, maintain TF infusion rate
Moderate Hx and/or physical evidence Order abdominal series X-rays to assess for small bowel obstruction. If SB, notify primary team. Stop TF infusion. Replace existing NG
Catheter
Moderate >24 hr or Severe Hx and/or physical evidence Stop TF infusion. Consider
TPN
Table: GI Complications Associated with Enteral Feedings
(Adults)
Diarrhea Mild 1-2 x per shift or
100-200cc/12 hrs
Maintain TF infusion rate. Evaluate for pharmaceutical causes. Increase to goal
Moderate 3-4 x per shift or
200-300cc/12 hrs
Maintain TF infusion rate. Re-examine in
6 hrs. if mild or moderate, continue to goal rate;
Evaluate medications
Severe ▼TF infusion rate by
50%. Order stool studies. Evaluate medications. Give antidiarrhea meds.
Table: GI Complications Associated with Enteral Feedings (Adults)
High NG output with post-pyloric
Feeding Tube
Placemen
High Gastric
Residuals with gastric Feeding
Tube placement
(measured) NG output >
800cc (with post-pyloric FT placement)
Hold tube feedings Check
Xray to verify post-pyloric feeding tube placement
(measured) Hold tube feedings for residuals greater than 200cc
Start prokinetic agent; Head of bed elevated 30 degrees when possible. Check for constipation
Constipation (measured) Less than 2 bowel movements per week
Stool softeners and water boluses
use fibre-containing feed avoid drugs containing sorbitol and Mg exclude and treat
Clostridium difficile infection
faecal impaction consider
malabsorption (pancreatic enzymes, elemental feed)
lactose intolerance (lactose-free feed)
using loperamide
WHEN TO USE TPN
Parenteral nutrition should only be used in patients with an inaccessible or nonfunctional gastrointestinal tract. Some of the most common reasons are due to a massive gastrointestinal bleed, acute abdomen, bowel obstruction/ileus, intractable vomiting or diarrhea, or prolonged
NPO status postoperatively—greater than 7 to 10 days. The potential for transitioning to enteral feedings should be reevaluated daily in patients on parenteral nutrition. The perfect time for such a reevaluation is during interdisciplinary ICU morning rounds
Early initiation of nutritional support is integral to the recovery of a critical illness, and evidence supports that enteral nutrition is both efficient and effective in providing necessary nutrition, particularly in the mechanically ventilated population. The key to obtaining nutrition in a timely manner is interdisciplinary collaboration among the critical-care physician, critical-care nurse, and nutritionist. This collaboration is best achieved through daily interdisciplinary rounds on all critically ill patients so that all team members have input into the patient's plan of care
overfeeding is
◦ useless - upper limit to amounts of protein and energy that can be used
◦ dangerous
hyperglycaemia and increased infection
uraemia
hypercarbia and failure to wean
hyperlipidaemia
hepatic steatosis
underfeeding is also associated with malnutrition and worse outcomes
carbohydrate
EN: oligo- and polysaccharides
PN: concentrated glucose lipid
EN: long and medium chain triglycerides
PN: soya bean oil, glycerol, egg phosphatides nitrogen
EN: intact proteins
PN: crystalline amino acid solutions water and electrolytes micronutrients
glutamine?
selenium?
immunonutrition?
EVALUATING NUTRITIONAL STATUS Although no single indicator provides an accurate depiction of a patient's nutritional status, parameters commonly used in all patients requiring a nutrition consult are body mass index (BMI), albumin/prealbumin level, nitrogen balance, and serum levels of trace elements
An initial nutritional assessment includes a physical assessment and medical history. BMI is a common anthropometric measure of nutritional status used to diagnose obesity and under nutrition associated with clinical conditions.
A common method of measuring a patient's protein status is the serum albumin level. Studies show that critically ill patients receiving long-term ventilation have low albumin levels during their hospitalization. These low levels are likely a reflection of both nutritional status and prolonged physiological stress associated with illness and/or ventilator weaning .
Many practitioners, however, prefer to measure the prealbumin level, because albumin changes in response to outside factors such as sepsis and surgery. Serum levels of prealbumin have a half-life of three to five days compared with 21 days for albumin. The rapid turnover of prealbumin is a reflection of its increased sensitivity to change in a body's protein status, therefore making it a more immediate indicator of physiological stress and nutrition status.
Decreased protein intake depletes the body's nitrogen reserve, which is manifested as a negative nitrogen balance. A patient's nitrogen balance is calculated by measuring the amount of urea nitrogen excreted in urine over 24 hours and utilizing a standard formula, and then comparing that to the amount of protein ingested during that same 24-hour period.
N Balance(g)=(protien intake (g)/6.25)-(UUN+4)
4=Daily nitrogen loss in grams ,other than UUN.BUT if UUN is>30g/24h,add 6 =Daily nitrogen loss in grams.
Serum levels of the trace elements of magnesium and phosphorus are biochemical indicators routinely used by clinicians to monitor nutritional status in critically ill patients.
Magnesium deficiencies can be associated with acute diarrhea, a potential side effect of enteral feedings.
Magnesium and phosphorus are important in energy synthesis and wound healing. Furthermore, abnormal levels of either of these electrolytes can cause cardiac, neurological, and neuromuscular disorders.
Step1: Weight Calculation
Ideal Body Weight (IBW) will be used for nutritional estimates for the majority of patients
Use Lean Body Weight (LBW) if pt obese or edematous a. IBW=50 kg + or 45 kg for females (2.3 kg for each inch over
60") b. Lean Body Weight=IBW + 0.4 (Actual weight - IBW)
Step 2: Non-protein Energy calculations
25-30 kcal/kg {aim for 25Kcals/kg}
65-70% as carbohydrate and 25-30% as fat
Consider 20 % calories reduction if pt is sedated or paralyzed
Step 3: Protein requirement calculations
1.5 – 1.8 g/kg/d utilizing IBW for all patients
Severe Hepatic Encephalopathy: protein restrict to 0.6 g/kg /d
Renal failure: i. If patient on RRT, full protein supplementation at 1.5 –2.5 g/kg/d should be instituted. ii. If not on RRT, consider reducing initial protein intake to 1 – 1.3 g/kg/d and follow BUN daily. iii. If patient oliguric, start at ≤ 0.6 g/kg/d and follow daily BUN
Step 4: Micronutrient calculation
Daily Electrolyte requirement
Daily Vitamins requirement
Daily Trace Element requirement
Daily requirement of fluid losses from GIT
(See the following table)
Sodium 1.0 mmol/kg/day
Potassium 1.0 mmol/kg/day Dependent on renal function
Phosphate 0.2 mmol/kg/day Dependent on renal function
Magnesium 0.3 mmol/kg/day Dependent on renal function
1.
2.
3.
Calcium 0.1 mmol/kg/day
Vitamins groups B daily.B12, Folate, A, D, E, K weekly Trace elements as required
Replacement solutions
Urine 1.½ Normal saline ± KCl 10 ml/L
2. Nasogastric/ileostomy 2. ½ Normal saline ± KCl 10 ml/L
3. Pancreatic/biliary fistulae 3. Ringer Lactate or Acetate
Canadian Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. J. of Parenteral Enteral Nutr. 27: 355–73
1.Fluid
2. Energy kcal/kg/day
30-40 ml/kg BW
3. Indirect calorimetry
3. Protein Normal prot : 0.8 g/kg/d HD. CVVHD : 1.1 –
1.4 g/kg/d Sepsis/trauma : 1.2 – 2.0 g/kg/d Severe burns : 2.5 – 4.0 g/kg/d
energy - 25 kCal/kg/day (ACCP)
NUTRITIONAL REQUIREMENTS
BEE classically is estimated by the Harris- Benedict equation:
For men, BEE = 66.5 + (13.75 x kg) + (5.003 x cm) -
(6.775 x age)
For women, B.E.E. = 655.1 + (9.563 x kg) + (1.850 x cm) - (4.676 x age)** BEE - Basal Energy Expenditure
Total Energy Expenditure ( TEE) = BEE x Injury Factor
Injury Factor
Mild illness 1 – 1.25 eg. minor op 1.2
Moderate illness 1.25 – 1.5 eg skeletal trauma 1.35
Severe illness 1.5 – 1.75 eg major sepsis 1.60
Estimated Total Energy Requirement = BEE x
Activity Factor x Injury Factor
INDIRECT CALORIMETRY
Most accurate.
Portable bedside system measuring of EE and resp quotient by measuring and analysing the O2 consumed (
VO2) and the CO2 expired ( VCO2)
REE(Kcal/24hr)=(3.9ΧVo2)+(1.1 Χ Vco2)-61
Respiratory Quotient = CO2 production/O2consumption
RQ Interpretation> 1.00 overfeeding
0.9 – 1.00 CHO oxidation
0.8 – 0.9 Mixed nutrients oxidation
0.7 – 0.8 Fat and protein oxidation
Limitations: expensive equipment , need trained personnel,O2 sensor is not reliable at inspired O2 levels above 50% so it is unreliable in pts with respiratory failure who require inhaled O2 concentrations above 50%.
nitrogen
◦ no benefit from measuring nitrogen balance
◦ nitrogen 0.15-0.2 g/kg/day
◦ protein 1-1.25 g/kg/day
◦ severely hypercatabolic patients (eg burns) may receive up to 0.3 g nitrogen/kg/day
Add 2-2.5ml/kg/day of fluid for each degree of temperature
Account for excess fluid losses
Adequate electrolytes, micronutrients, vitamins
Avoid overfeeding
Obesity: feed to BMR, add stress factor only if severe i.e. burns/trauma
Carbohydrate, CHO
Main source of energy, 60% of total energy requirement.
2-3 g/Kg/day 1 g CHO = 4 Kcal
Fat
30-40% of caloric intake.
1.5-2 g/Kg/day 1 g Fat = 9 Kcal
Protein
Not a major energy source. Provide essential & non essential amino acids for protein synthesis. Use as energy substrate (CHO
@ Fat precursor) in excess.
1-1.5 g/Kg/day
1 g Protein = 4 Kcal.
1 g N2 = 6.25 g Protein.
Enteral feeding should be started early within the first 24–48 hours following admission. The feedings should be advanced toward goal over the next 48–72 hours
Efforts to provide >50% to 65% of goal calories should be made to achieve the clinical benefit of EN over the first week of hospitalization.
If unable to meet energy requirements (100% of target goal calories) after 7 days by the enteral route alone, consider initiating supplemental PN
PN should not be initiated in the immediate postoperative period, but should be delayed for 5-7 days
If there is evidence of protein-calorie malnutrition at admission and EN is not feasible, it is appropriate to initiate PN as soon as possible following admission and adequate resuscitation
The use of additional modular protein supplements is a common practice, as standard enteral formulations tend to have a high non protein calorie : nitrogen ratio
In the setting of hemodynamic compromise (patients requiring significant hemodynamic support, including high-dose catecholamine agents, alone or in combination with large volume fluid or blood product resuscitation to maintain cellular perfusion),
EN should be withheld until the patient is fully resuscitated and/or stable
In patients with body mass index (BMI) <30, protein requirements should be in the range of 1.2–2.0 g/kg actual body weight per day, and may likely be even higher in patients with In the critically ill obese patient, permissive underfeeding or hypocaloric feeding with
EN is recommended. For all classes of obesity where BMI is >30, the goal of the EN regimen should not exceed 60% to 70% of target energy requirements or 11–14 kcal/kg actual body weight/day (or
22–25 kcal/kg ideal body weight/day)
Protein should be provided in a range ≥ 2.0 g/kg ideal body weight/day for class I and class II patients (BMI 30–40), ≥ 2.5 g/kg ideal body weight/day for class III (BMI ≥40) burn or multiple trauma
Use of chlorhexidine mouthwash twice a day should be considered to reduce risk of ventilator-associated pneumonia.
Patients with acute respiratory distress syndrome and severe acute lung injury should be placed on an enteral formulation characterized by an antiinflammatory lipid profile (i.e., omega-3 fish oils, borage oil) and antioxidants
Specialty high-lipid low-carbohydrate formulations designed to manipulate the respiratory quotient and reduce CO2 production
ARE NOT RECOMMENDED for routine use in ICU patients with acute respiratory failure.
Patients receiving hemodialysis or continuous renal replacement therapy should receive increased protein, up to a maximum of 2.5
g/kg/day
Protein should not be restricted in patients with renal insufficiency as a means to avoid or delay initiation of dialysis therapy.
Standard enteral formulations should be used in ICU patients with acute and chronic liver disease. The branched chain amino acid formulations should be reserved for the rare encephalopathic patient who is refractory to standard therapy with luminal-acting antibiotics and lactulose
Patients with mild to moderate acute pancreatitis do not require nutrition support therapy (unless an unexpected complication develops or there is failure to advance to oral diet within 7 days)
Patients with severe acute pancreatitis may be fed enterally by the gastric or jejunal route
In acute pancreatitis change the content of the EN delivered from intact protein to small peptides, and long-chain fatty acids to medium-chain triglycerides or a nearly fat
Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care
Medicine and American Society for Parenteral and Enteral Nutrition
Crit Care Med 2009 Vol. 37, No. 5
HOW TO CALCULATE TPN ?
Steps Example:
A 56 yr, 1.75 m tall, 70 kg man
1. Determine the protein 70 kg x 1.5g/kg/d = 105 g/d ( = requirement
16.8g N)
2. 2. Determine the total caloric Using Harris Benedict equation: requirement
BEE = 66 + ( 13.7 x 70kg) + ( 15 x 175cm) – (6.8 x 56 yr) = 1519 kcal/day ( round off to 1500 kcal/day) TEE = BEE x IF = 1500 x 1.3 =
1950 kcal/day
3. Divide the total caloric If ratio 60:40 requirement between two energy
1950 x 0.6 : 1950 x 0.4 = 1170 : substrate, CHO : fat ( 60:40 or 780
70:30
4. Determine calorie : nitrogen ratio 1950 : 16.8 = 116 : 1
5. Calculate amount of CHO needed If using 70% dextrose solution ( 100 ml provide 70 g CHO x 3.4 kcal/g = 238 kcal) 1170 kcal / 238 kcal x 100 mls =
492 mls ~ 500 mls
6. Calculate amount of fat emulsion If using 20% intralipid ( provides 2needed kcal/ml) 780 kcal divide into 2 kcal/ml = 390 ml
7. Estimate fluid requirement 40 ml/kg/day x 70 kg = 2800 ml/d Therefore :
2800 – ( 500 + 390) = 1910 ml ( of water to be added to meet fluid requirement )
.
8
8.Order electrolytes: Na+, K+,Mg2+, Ca, phosphorus, acetate and chloride
9. Order multivitamin, trace minerals and vitamin K if needed
10. Determine flow rates : volume / 2800 ml / 24H = 117 ml/H24h
catheter-related sepsis
Catheter Malposition pneumothorax hydrothorax
Arterial puncture
Metabolic
Hyperglycaemia Hypoglycemia if TPN is abruptly stopped
Increased CO2 production & increased O2 consumption if infusion rates beyond 4 ml/kg/mt. Hypomagnesemia, hypophosphatemia if not supplemented hyperchloraemic metabolic acidosis electrolyte imbalance - low Pi, K, Mg refeeding syndrome abnormal LFTs Fatty liver deficiency of thiamine, vit K, folate
Clinical studies have shown that ICU patients receiving parenteral nutrition have demonstrated a higher incidence of metabolic and infectious complications than those patients receiving enteral nutrition.
Common metabolic complications include hyperglycemia, hypoglycemia, and refeeding syndrome — the body suddenly shifts from fat metabolism to carbohydrate metabolism. This shift causes a surge in insulin levels, which in turn leads to an increase in the cellular uptake of phosphorus, resulting in hypophosphatemia.
Primary symptoms of hypophosphatemia are muscle weakness and wasting and general fatigue, all of which are barriers to successful ventilator weaning and healing.
Acute cholecystitis is a common complication of parenteral nutrition, related to the complete lack of usage of the gastrointestinal tract resulting in biliary stasis in the gall bladder.
Total parenteral nutrition must infuse through a central venous catheter, and a bacterial infection of that catheter is a serious and potentially life-threatening risk.
glutamine?
selenium?
immunonutrition?
primary fuel for enterocytes, lymphocytes and neutrophils; also involved in signal transduction and gene expression massive release from skeletal muscle during critical illness may then become ‘conditionally essential’ is not contained in most TPN preparations
reduces villus atrophy in animals and humans reduced pneumonia and bacteraemia in two studies - multiple trauma, sepsis one much larger study (unselected ICU patients) showed no effect difficult to give adequate dose enterally
Liverpool study in ICU showed reduction in late mortality
London study of all hospital TPN patients showed no benefit
French trauma study showed reduced infection but no mortality effect
German ICU study improved late survival in patients fed for more than 9 days
glutamine becomes conditionally essential in critical illness and is not given in standard TPN parenteral supplementation appears to be beneficial in patients requiring TPN for many days
regulates free-radical scavenging systems low levels common in ICU patients several small studies inconclusive but suggest benefit one large, flawed recent study showed nonsignificant mortality benefit
omega-3 fatty acids
produce less inflammatory eicosanoids arginine
nitric oxide precursor
enhances cell-mediated immunity in animals nucleotides
DNA/RNA precursors
deficiency suppresses cell-mediated immunity
few studies in ICU populations some found reduced infection in elective surgery one unblinded study has shown reduced mortality in unselected ICU patients; benefit in least ill (CCM 2000; 28:643) another showed increased mortality on re-analysis which barely failed to reach statistical significance (CCM 1995;
23:436)
first meta-analysis (Ann Surg 1999; 229:
467)
no effect on pneumonia
reduced other infections and length of hospital stay
increased mortality only just missing statistical significance
did not censor for death second meta-analysis (CCM 1999; 27:2799)
reduced infection
reduced length of ventilation and hospital stay
no effect on mortality
third meta-analysis (JAMA 2001; 286:944)
benefit in elective surgery
increased mortality in ICU patients with sepsis large Italian RCT (ICM 2003; 29:834)
compared enteral immunonutrition with TPN
stopped early because interim analysis showed increased mortality in septic patients
44.4% vs 14.3%; p=0.039
6.
7.
8.
1.
2.
3.
4.
5.
9.
10.
Enumerate the Effects of Malnutrition?
Mention the Consequences of malnutrition?
When should we start to feed patient admitted to ICU ?
Mention the complications of enteral tube feeding ?
How to mange diarrhoea in COPD patient on mechanical ventilation received entral feeding through nasogastric tube ?
WHEN to use TPN ?
What should the feed contain in ICU ?
HOW to calculate TPN in60 yr, 1.75 m tall, 80 kg female ?
Mention the Limitations of indirect calorimetry ?
Mention the complications of TPN ?