EX-PRESS ® Glaucoma Filtration Device
advertisement
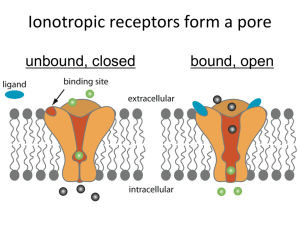
Glaucoma Surgery ® & The EX-PRESS Device Ike K. Ahmed, MD EXP11732SK EX-PRESS® Device Brief Statement CAUTION: Federal law restricts this device to sale by or on the order of a physician. INDICATION: The EX-PRESS® Glaucoma Filtration Device is intended to reduce intraocular pressure in glaucoma patients where medical and conventional surgical treatments have failed. GUIDANCE REGARDING THE SELECTION OF THE APPROPRIATE VERSION: Prior clinical studies were not designed to compare between the various versions of the EX-PRESS® Glaucoma Filtration Device. The selection of the appropriate version is according to the doctor's discretion. CONTRAINDICATIONS: The use of this device is contraindicated if one or more of the following conditions exist: · Presence of ocular disease such as uveitis, ocular infection, severe dry eye, severe blepharitis. · Pre-existing ocular or systemic pathology that, in the opinion of the surgeon, is likely to cause postoperative complications following implantation of the device. · Patients diagnosed with angle closure glaucoma. WARNINGS/PRECAUTIONS: · The surgeon should be familiar with the instructions for use. · The integrity of the package should be examined prior to use and the device should not be used if the package is damaged and sterility is compromised. · This device is for single use only. · MRI of the head is permitted, however not recommended, in the first two weeks post implantation. ATTENTION: Reference the Directions for Use labeling for a complete listing of indications, warnings, precautions, complications and adverse events. EXP11706SK EXP11732SK Disclosures • Consultant (+S) – – – – – – – – – – – – – Alcon Allergan Aquesys AMO Carl Zeiss Clarity Endooptiks Eyelight Glaukos iScience Ivantis Pfizer Transcend • Research Grants • Speaker Honoraria (S) – – – – – – – – – Alcon® Allergan Aquesys Carl Zeiss iScience Merck Pfizer SOLX Visiogen – New World Medical Ike K. Ahmed EXP11732SK Glaucoma Surgical Options EXP11732SK Glaucoma Surgery Trends • Evolutionary improvements in trabeculectomylike procedures – Canaloplasty – EX-PRESS® glaucoma filtration device • Increased use of long-tube shunts – Ahmed, Baerveldt glaucoma drainage devices • New field “Minimally Invasive Glaucoma Surgery (MIGS)” – Safe, quick procedures with modest IOP-lowering – Use at time of cataract surgery 5 Ike K. Ahmed, MD EXP11732SK Overview of Current MIGS Procedures Commercialized and in Development Commercialized • Trabectome • ECP Schlemm’s Canal Cycloablation •Not FDA Approved 6 •† Trademarks are the property of their respective owner. Investigational* • • • • • iStent† Hydrus ELT Cypass Aquesys Schlemm’s Canal Suprachoroidal Space Subconjunctival Space Ike K. Ahmed, MD EXP11732SK Ab-Interno MIGS Pathways Subconjunctival Schlemm’s Canal Suprachoroidal IOP Drop More Potent Moderate Moderate Risk Slightly More Lowest Risk Low Ease of Use Easy to Perform +/- Gonio Somewhat more Difficult Requires Gonio View Easiest to Perform +/- Gonio Potential Issues ?Bleb Issues ?Episcleral Healing ?Hypotony ?Uncertainty of Placement ?EVP Floor ?Distal Outflow Status ?Angle Bleeding ?Variable IOP Drop ?Fibrosis in SCS Other Features Familiarity Ability to modulate postop healing Physiologic ?Titratable Potential Ready for Primetime? EXP11732SK Patient Profiles: New Procedures Trab-type Procedures EX-PRESS® Device • Moderate-advanced disease • Progressing normal pressure glaucoma • Open Angle • Low IOP target (i.e., <13mmHg) • Intolerant to meds and failed SLT/ALT Source: EX-PRESS® glaucoma filtration device package insert 8 Ike K. Ahmed, MD EXP11732SK Glaucoma Surgery • Has traditionally been all about efficacy • Serious safety issues have promoted evolutionary improvements 9 Ike K. Ahmed, MD EXP11732SK Evolution of the Guarded Filtration Procedure • Wound healing strategies • Suture tension & laser suture lysis • Fornix-based flaps • Non-penetrating approaches • EX-PRESS® glaucoma filtration device 10 Ike K. Ahmed, MD EXP11732SK What Differentiates one Filter from the Next in My Experience Intraoperative • • • • AC shallowing Tissue trauma Bleeding Length of procedure Postoperative • • • • • • • • • • Hypotony Shallow/flat AC Choroidals Hyphema Bleb leak Bleb encapsulation Bleb dysthesia IOP control Visual recovery Postop interventions EXP11732SK EX-PRESS® Glaucoma Filtration Device A Limbal Aqueous Device • Made of rigid 316LVM stainless steel – same as cardiac stents • < 3mm long • Internal lumen size – 50µm/200µm • Biocompatible • MRI of the head is permitted, however not recommended, in the first two weeks post implantation. Source: EX-PRESS® glaucoma filtration device package insert P-50 A Nyska, Y. Glovinsky, M. Belkin, and Y. Epstein. Biocompatibility of the EX-PRESS® miniature glaucoma drainage implant. J Glaucoma. 2003 Jun; 12(3):275-80 EXP11732SK EX-PRESS® Device = Trabeculectomy • Potent IOP lowering1 • Requires scleral flap for additional flow control – Although not as critical • Requires functioning bleb, control of episcleral fibrosis – Conjunctival health a factor – Wound healing modulation 1) P. J. G. Maris, K Ishida, P A Netland. Comparison of Trabeculectomy with EX-PRESS® miniature Glaucoma Device Implanted Under Scleral Flap. J Glaucoma. 2007 Jan; 16:14-19. 13 Ike K. Ahmed, MD EXP11732SK EX-PRESS® Device > Trabeculectomy • Quieter eyes in early postoperative period1 • Avoidance of intraoperative malignant glaucoma or choroidals1 • Reduction of early postoperative hypotony1 • No iridectomy required • Intraoperative maintenance of anterior chamber • Additional fluidic restriction (50um lumen) • Consistency 1) P. J. G. Maris, K Ishida, P A Netland. Comparison of Trabeculectomy with EX-PRESS® miniature Glaucoma Device Implanted Under Scleral Flap. J Glaucoma. 2007 Jan; 16:1419. 14 Ike K. Ahmed, MD EXP11732SK EX-PRESS® Device Rationale & Transition • • • • • • • • Hit low IOP target1,2 Enhanced predictability1 Minimize tissue disruption Improved safety1 Quieter postoperative course1 Quicker visual recovery2 Reduction of postop visits2 Improved bleb morphology2 1) Maris PJ et al., J Glaucoma 2007 2) Good TJ, Kahook MY, AJO 2011 15 Ike K. Ahmed, MD EXP11732SK On-Label Indications (US) • Open angle glaucoma • Failed medical and laser/surgical therapy • Anatomical factors – Scleral thickness – Angle anatomy 16 Ike K. Ahmed, MD EXP11732SK EX-PRESS® Device Technique Pearls • • • • Anatomical landmarks Scleral flap design and thickness Device entry and angulation Postoperative bleb management Ike K. Ahmed, MD EXP11732SK Surgical Limbal Anatomy Cornea Blue-zone Scleral Spur Sclera EXP11732SK AC Entry Entry should be just at anterior aspect of scleral spur, at posterior aspect of the limbal blue zone. EXP11732SK Planning Scleral Flap Position & Size EXP11732SK Identify Surgical Limbus Planned entry point for EX-PRESS® Device EXP11732SK EXP11732SK 3.5x2.5 mm Scleral Flap 1/2mm anterior gap to prevent excessive device compression 1.0 0.5 1.0 1.0 2.5 3.5 Ensure adequate flap overlap lateral and posterior to EX-PRESS® device to allow control of aqueous flow EXP11732SK EXP11732SK Enter at anterior scleral spur/posterior blue zone Parallel to iris plane - aided by rotation of eye downwards EXP11732SK Parallel to Iris EXP11732SK EXP11732SK Postop Management • • • • Bleb management Laser suture lysis Needling Steroids 28 Ike K. Ahmed, MD EXP11732SK EX-PRESS® Glaucoma Filtration Device • An evolutionary improvement in trabeculectomy • Smaller incision, more standardized • Patient selection is much the same, although with improvement in safety and reproducibility, may be slightly broader (earlier intervention) • Retains high efficacy 29 Ike K. Ahmed, MD EXP11732SK