Presentazione standard di PowerPoint
advertisement

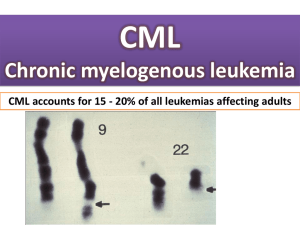
Targeted therapy: Falso mito o reale innovazione? Stefano Iacobelli Cancer Clinic & Laboratory of Molecular Oncology Consorzio Interuniversitario Nazionale per la BioOncologia (CINBO) What are Targeted Therapies? Targeted cancer therapies are drugs or other substances that block the growth and spread of cancer by interfering with specific molecules involved in tumor growth and progression. Targeted cancer therapies that have been approved for use in specific cancers include drugs that interfere with cell growth signaling or tumor blood vessel development, promote the specific death of cancer cells, stimulate the immune system to destroy specific cancer cells, and deliver toxic drugs to cancer cells. NCI www.cancer.gov Mechanisms of common Targeted Anticancer Therapies: mAbs and TKIs Langer C & Soria JC, Clin Lung Cancer 11: 82-90, 2010 FDA approved TKIs and mAbs for cancer therapy aAgents with antiangiogenic mechanism Li J et al., Targ Oncol 2012 Myth: Imatinib Mesylate, “Proof of Principle” for Targeted Therapy • Imatinib Mesylate targets the bcr-abl TK very specifically. • Bcr-abl is the root cause of CML, essentially a “monogenetic disease” Before Imatinib, only 30% of patients with CML survived for even five years after being diagnosed. Results with Imatinib as initial therapy for newly diagnosed chronic-phase CML 5-yr CCR: 87% 5-yr OS: 89% Druker BJ et al., N Engl J Med 2006 The Reality: Targeted Therapy in the Common Solid Tumors CML and Breast Cancer Targeted Therapies Vary in Effectiveness: The role of the “TARGET” CML Patients All Breast Cancer Patients HER2 + Breast Cancer Patients Imatinib Trastuzumab Trastuzumab ~ 90% Response < 10 % Response ~ 35% Response The Ideal Target Driving mutation in a “ Dumb ” tumor that is Easily druggable and the mutation is really common Stupid and Smart Cancers Stupid Cancers Smart Cancers • Single dominat mutation • Multiple mutational drivers • Small mutational load • Large mutational load • Monotherapy is effective • • Multi-targeted therapy required Resistance rare, late, same pathway • Resistance common, early Sledge G, ASCO 2009 CML: A Stupid Cancer NSCLC: A Smart Cancer One mutation for every 3 cigarettes! A= Inter- and intra-chromosomal rearrangements B= LOH and allelic imbalance C= Copy number variations D= Single nucleotide variants • Driven by a single chromosomal translocation (BCR-ABL) • Success with the first drug that came along • That doesn’t work? - Use an "ib" targeting the same kinase domain Red = amplification Purple = LOH Black = mutation Lee et al., Nature 465: 473-7, 2010 Somatic mutations of the EGFR gene were found in 15 of 58 unselected tumors from Japan and 1 of 61 from the US Somatic mutations were identified in the tyrosine kinase domain of the EGFR gene in 8 of 9 patients with gefitinib-responsive lung cancer, as compared with none of the seven patients with no response (P<0.001) Erlotinib vs. standard chemotherapy as first-line treatment for European pts with advanced EGFR-mutation positive NSCLC - EURTAC phase III study 174 pts with EGFR mutations (exon 19 deletion or L858R mutation in exon 21) enrolled mPFS 9.7 vs. 5.2 months Erlotinib 150 mg daily Standard chemotherapy: cisplatin 75 mg/m(2) d1 + docetaxel 75 mg/m(2) d1 or gemcitabine 1250 mg/m(2) d 1,8 q21 Rosell R et al., Lancet Oncol 2012; 13:239-46 Gefitinib in Refractory Advanced NSCLC No Benefit Thatcher N et al., Lancet 366: 1527-37, 2005 40% K-Ras mutations in CRC No difference K-Ras mutations and Benefit from Cetuximab in Advanced CRC mOS 9.5 vs 4.8 months Karapetis CS, N Engl J Med 359:1757-65, 2008 Clinical evidence of oncogene addiction The need of a “COMPANION DIAGNOSTIC TEST” Nagahiro Saijo, Cancer Res Treat. 2012; 44:1-10 Recently, 2 targeted therapies were approved by FDA with a companion diagnostic to identify enriched subpopulations of patients that are more likely to respond to the drug Parkinson DR et al., Clin Cancer Res 2012 Diagnosis of an EML4-ALK-Positive NSCLC in a single patient Adenocarcinoma (H&E) Kwak EL et al., N Engl J Med 2010 IHC analysis of ALK in tumor cells (brown) RT-PCR of EML4-ALK Panel A: The green probe hybridizes to the region immediately 5’ to ALK, and the red probe to the 3’ region. The separation of red and green probes indicates a chromosomal rearrangement of ALK. The probe used was the Vysis LSI ALK Dual Color, Break Apart Rearrangement Probe (Abbott Molecular) Response to ALK Inhibition and PFS ORR 60.8% Crizotinib 250 mg bid in 28-day cycles mPFS 9.7 months CT before and after Crizotinib Kwak EL et al., N Engl J Med 2010; Camidge DR et al., Lancet Oncol 2012 Chapman PB et al., N Engl J Med 2011 Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation RR 48% vs. 5% Vemurafenib vs. Dacarbazine Vemurafenib 960 mg orally bid Dacarbazine 1000 mg/mq d1 q21 BC – Using usual selection criteria (EBCTCG) (100 N0, pre-menopausal pts receiving CT, after 5 yrs follow-up) 100 83.5 will be alive even w/o CT 13.5 will die despite CT 3 will be alive thanks to CT 75 50 Using the 70-gene signature Only 27% of pts will be overtreated 25 0 Conclusions The outcome of CML was transformed by targeted therapy: median survival increased from about 4 yrs to 20-25 yrs Solid tumors are more complex than CML Therefore the outcomes in CML are an aberration, and we are not likely to see such a transformation in outcomes in solid tumors Only a co-development strategy that identifies biomarkers of response and treats only vulnerable tumors will be defensible going forward Development strategies that administer therapies to populations that are not selected or are selected with methodologies not validated, must become a strategy of the past. Molecular Diagnostics: The next step Stefano Iacobelli Cancer Clinic & Laboratory of Molecular Oncology Consorzio Interuniversitario Nazionale per la BioOncologia (CINBO) Targeted cancer drugs are expensive and often fail to yeld clinical benefit NSCLC Breast Pancreas Breast Provocative statement 1: Drug companies cannot afford NOT to have a molecular diagnostic division Tissue samples for MDx will become ever smaller as diagnosis improves, requiring concentration of MDx testing in single large central services laboratories These MDx companies that offer the broadest range of diagnostic tests will receive the samples available for MDx If co-development of drug and companion MDx test will become the norm and integral part of FDA/EMA regulatory process, any company that does not control the entire chain of events will be vulnerable. Provocative statement 2: Biomarker discovery should start before Phase 1 Too often, companies start to think about biomarkers when the drug has falled in phase 3 (the Iniparib scenario) Candidate biomarkers of response should be identified while a compound is in research phase, validated in phase 1 and 2 trials and used as an enrollment criterion in phase 3 Provocative statement 3: No tissue, no trial! Is it ethical to make biopsies mandatory for participation in trials or should we realize high consent to biopsies through patient education? Provocative statement 4: Heathcare insurers will not pay for Rx without companion Dx in the future Future drug reimbursement will depend on quality of life years gained How much are payers willing to pay for a MDx test that rules out half of the patient population for a € 100,000 targeted therapy?