jean-guy baril md clinique médicale du quartier latin

Innovative Strategies for the Management of HIV Infection
Dual therapies without NRTIs
Jean-Guy Baril, MD
Clinique médicale du Quartier Latin
CHUM
This activity is supported by an educational grant from:
Disclosures
Dr. Jean-Guy Baril
Received consultant, investigator or speaker honoraria/grants from the following companies
•
AbbVie
•
Bristol-Myers Squibb
•
GlaxoSmithKline
•
Boehringer Ingelheim
•
Pfizer
•
Roche
•
Tibotec
•
Merck Frosst
•
Gilead
ANTIBODY Healthcare Communications received an unconditional grant from
AbbVie Canada for the literature review
Objectives
• Review the data from studies supporting the use of dual therapy on treatment-naive or experienced patients with an undetectable viral load.
• Know the studies done with a protease inhibitor in combination with another single agent such as an integrase inhibitor, maraviroc, 3TC or an NNRTI.
• Discuss the role of these options in clinical practice.
Denis’ Case
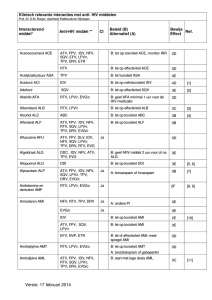
• Patient has never been treated for his HIV. Suffers from diabetes but is well controlled. He also has renal insufficiency.
• Accepted to begin treatment because of a drop in his CD4 to 370 and a viral load of 150 000
Lab results:
• Creatinine: 133; eGFR: 55; Urinalysis: N; MAU: 4.6 mg/mmol
(N<2.1)
• HLAB5701: Positive for genotype: no mutation.
• HBsAG negative, anti-HBs positive, anti-HBc negative, anti-
HCV negative
Which treatment to start with?
• 1) Atripla
• 2) Truvada + PI/r
• 3) Kivexa + Efavirenz
• 4) Combivir + Efavirenz
• 5) PI/r + Raltegravir
• 6) PI/r + Efavirenz
The ongoing need for novel regimens
“ A person starting combination therapy can expect to live about 43 years at 20 years of age… ”
The Lancet, 2008 1
As patients live longer on therapy… new therapeutic options which lessen the impact of
ARV therapy on their bodies are especially important.
What are the goals of NRTI-free therapy?
2
•
Maintain efficacy
•
Prevent resistance
•
Reduce toxicities
•
Maintain and improve adherence
•
Reduce costs
(including the total cost of care)
1.
The Antiretroviral Therapy Cohort Collaboration. Lancet 2008;372:293-99;
2.
2. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents.
Department of Health and Human Services
NRTI-sparing approach:
Findings to date
•
Initial studies showed
1
…
• higher rates of treatment failure poorer tolerability
(i.e. DMP-006; IDV+EFV)
Recent (2011) meta-analysis of 10 PI monotherapy trials showed
2
…
• increase in the risk of virologic failure
• decrease in viral suppression
1. Staszewski, S., et al. Efavirenz plus Zidovudine and Lamivudine, Efavirenz plus Indinavir, and Indinavir plus Zidovudine and Lamivudine in the
Treatment of HIV-1 Infection in Adults.
New England Journal of Medicine, 1999. 341 (25): pp. 1865-1873.
2. Mathis, S., et al. Effectiveness of protease inhibitor monotherapy versus combination antiretroviral maintenance therapy: a meta-analysis.
PLoS One,
2011. 6 (7): p. e22003.0
Innovative strategies for HIV care
The working group:
• Dr. S. Walmsley, ON (Co-chair)
• Dr. J.G. Baril, QC (Co-chair)
• Dr. C. Murphy, BC
• Dr. J. Angel, ON
• Dr. J. Gill, AB
• Dr. G. Smith, ON
• Sandra Blitz, ON
What did the working group do?
Reviewed the current and available evidence on innovative dual therapies(PI/r + RAL or MVC or NNRTI or 3TC) for ARVnaïve and
-experienced patients.
Methods
•
Literature search using PubMed was done from 2002 through
February 2012
•
International AIDS Society Conference on HIV Pathogenesis and Treatment and Prevention (WAC/IAS) 2009-2011 and Conference on Retroviruses and
Opportunistic Infections (CROI) 2009-2012 abstracts search
•
Additionally, an expert review committee consisting of HIV specialists reviewed and rated all identified trials and was queried around their knowledge of any other potentially relevant studies in existence, including those cited in the reference lists of identified studies
Trial selection
INCLUSION CRITERIA
• randomized controlled or prospectively designed single-arm trials
• minimum duration 24 weeks
• naive or switch of virologically suppressed patients
• primary outcome of suppression of viral load, change in viral load or virologic failure was acceptable
– other virologic outcomes were acceptable as a primary endpoint, if they were supplemented by secondary endpoints which looked at the above criteria
• secondary outcome data were included if available (toxicities and-or co-morbidities outcome)
• considered acceptable: pilot/proof-of-concept studies, abstracts.
EXCLUSION CRITERIA
• Case reports, reviews, correspondences and research letters
•
Phase I trials, laboratory studies , pharmacokinetic/pharmacodynamic studies, retrospective or included patients who were ARV-experienced but not suppressed or were pediatric, pregnancy/pMTCT or co-infection studies
NRTI-sparing trials:
ARVnaïve and – experienced patients
Regimen
PI/r + RAL
PI/r + MVC
PI/r + 3TC
PI/r + NNRTI
ARVnaïve
PROGRESS (LPV/r + RAL)
ACTG 5262 (DRV/r + RAL)
RADAR (DRV/r + RAL)
SPARTAN (ATV + RAL)
CCTG 589 (LPV/r + RAL)
A4001076 (ATV/r + MVC)
VEMAN (LPV/r + MVC)
MIDAS (DRV/r + MVC
LOREDA (LPV/r + 3TC)
ACTG 5142 (LPV/r + EFV)
ARV-experienced
KITE (LPV/r + RAL)
ATLAS (ATV/r + 3TC)
A5116 (LPV/r + EFV)
NEKA (LPV/r + NVP)
PROGRESS:
LPV/r + RAL vs. LPV/r + TDF/FTC in ARVnaïve patients
LPV/r 400/100 mg BID
+ RAL 400 mg BID (n=101)
Screening
Week 48
Primary
Efficacy
Endpoint
Week 96
LPV/r 400/100 mg BID
+ TDF/FTC 300/200 mg QD
(n=105)
Met Primary Endpoint of Non-inferiority
Primary endpoint: plasma HIV-1 RNA <40 copies/mL at week 48 (FDA-TLOVR)
•
LPV/r + RAL=83.2%,
•
LPV/r + TDF/FTC=84.8%
Difference -1.6%, 95% exact confidence interval (CI) -12.0%, 8.8%; P=0.850,
Safety and tolerability were similar at week 48
* 3 subjects were randomized but not dosed
Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir (LPV/r) combined with raltegravir (RAL) or tenofovir/emtricitabine (TDF/FTC) in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses. 2012;[epub ahead of print].
PROGRESS:
Week 96 (TLOVR)
(88.9% obs)
(85.2% obs)
RAL-TDF/FTC= 3.7% (95% CI: -7.5%, 14.3%) obs
Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir (LPV/r) combined with raltegravir (RAL) or tenofovir/emtricitabine (TDF/FTC) in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses. 2012;[epub ahead of print].
PROGRESS:
Mean percent change from baseline to weeks 48 and 96 in body fat parameters
LPV/r + RAL and LPV/r + TDF/FTC groups were compared using one-way ANOVA
Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir (LPV/r) combined with raltegravir (RAL) or tenofovir/emtricitabine (TDF/FTC) in antiretroviral-naive subjects: 96-week results of the PROGRESS study. AIDS Res Hum Retroviruses. 2012;[epub ahead of print].
PROGRESS:
Mean percent change in bone mineral density analyzed using DXA through 96 weeks of treatment van Wyk J. Body fat distribution changes after 96 weeks of therapy with lopinavir/ritonavir (LPV/r) plus raltegravir (RAL) compared with LPV/r plus tenofovir/emtricitabine (TDF/FTC) in antiretroviral (ARV)-naive, HIV-1-infected subjects from the PROGRESS study. Presented at: 13th European AIDS
Conference; October 12-14, 2011; Belgrade, Serbia.
Proportion of Subjects with ≥5% Decrease from Baseline in Total Bone Mineral Density
PROGRESS Bone Mineral Density
July 14, 2011
Results from a Single Arm Study of Darunavir/Ritonavir Plus
Raltegravir in
TreatmentNaïve HIV-1-Infected
Patients (ACTG A5262)
Babafemi Taiwo et al. ,
Northwestern Univ.,
Chicago, IL, US
Presented as poster no 551 at CROI
Year 2011
ACTG A5262
ACTG 5262 Aims, method and design
Aim
• To assess the efficacy and safety of DRV/r plus RAL in antiretroviralnaïve subjects
Methods and design
• A multicentre, single arm, open label, 52-week pilot study of RAL 400mg
BID plus DRV/r 800mg/100mg QD
RADAR study: Raltegravir combined with boosted Darunavir has similar safety and antiviral efficacy as tenofovir/emtricitabine combined with boosted darunavir in antiretroviral-naive patients
Author, R. Bedimo et al.
VA North Texas Health
Care System, Medicine, Dallas, United States
Presented as poster no MOPE214 at the 6 th
IAS conference, Rome
Year 2011
Radar study
RADAR study, 24 weeks : Key Findings
Key Findings
Outcome RAL with
DRV/RTV
Undetectable viral load, (% < 50)
88.9 %
Change in CD4 cell count
+123
TDF/FTC with
DRV/RTV
81.0 %
+174
P value
0.41
0.19
Other outcomes
Mean TC conclusion
+21.6
+8.8
Similar safety and efficacy
Working Group on Innovative Strategies for HIV Care, 2012.
0.20
The SPARTAN study: a pilot study to assess the safety and efficacy of an investigational NRTI- and RTV-sparing regimen of atazanavir (ATV) experimental dose of 300mg BID plus raltegravir (RAL) 400mg BID (ATV+RAL) in treatmentnaïve HIV-infected subjects
M.J. Kozal et al.
Yale University School of Medicine and VA CT Healthcare System, New Haven, United States
Presented as LB no : THLBB204 at the
XVIII th IAC conference, Vienna
Year 2010
The SPARTAN study
Atazanavir/r + TDF/FTC or Maraviroc in
Treatmentnaïve Patients (Study A4001078)
Open-label, 96-week Phase 2b Pilot Study
Randomization
1 : 1
N = 121
FTC/TDF + ATV/r (300/100 mg QD)
MVC (150 mg QD) + ATV/r (300/100 mg QD)
Screening
(6 weeks)
0 16 wk
24 wk
48 wk
Primary
Endpoint
• Primary Patient Eligibility Criteria:
− R5 HIV at screening
− HIV1 RNA ≥1,000 copies/mL
− CD4 ≥100 cells/mm 3
− No evidence of resistance to ATV/r, TDF, or FTC
Mills T, et al, 19th IAC; Washington, DC; July 22-27, 2012; Abst. TUAB0102.
96 wk
A4001078: Virologic Outcomes
82.0%
67.8%
ITT, NC=F
•
MVC arm: 6/8 with detectable viremia at week 96 had VL <250 cps/mL
•
No genotypic, phenotypic resistance or tropism changes detected in any failing subjects
Mills T, et al, 19th IAC; Washington, DC; July 22-27, 2012; Abst. TUAB0102.
A4001078: Adverse Events and Safety
Any AE, n (%)
Serious AE, n (%)
Grade 3 or 4 AE, n (%)
Discontinued due to AE, n (%)
MVC + ATV/r n=60
58 (96.7)
13 (21.7)
32 (53.3)
2 (3.3)
FTC/TDF + ATV/r n=61
61 (100.0)
11 (18.0)
20 (32.8)
0
Hyperbilirubinemia, n (%)
AE-related
Grade 3 or 4 AE-related
Grade 3 or 4 laboratory
Jaundice, n (%)
AE-related
Grade 3 or 4 AE related
18 (30.0)
10 (16.7)
42 (70.0)
16 (26.2)
8 (13.1)
34 (55.7)
10 (16.7)
5 (8.3)
6 (9.8)
1 (1.6)
Mean change in creatinine clearance from baseline to week 96:
• MVC + ATV/r = ↓1.5 mL/min
• TDF/FTC + ATV/r = ↓21.5 mL/min
Mills T, et al, 19th IAC; Washington, DC; July 22-27, 2012; Abst. TUAB0102.
NRTI-sparing trials:
ARVnaïve and – experienced patients
Regimen
PI/r + RAL
PI/r + MVC
PI/r + 3TC
PI/r + NNRTI
ARVnaïve
PROGRESS (LPV/r + RAL)
ACTG 5262 (DRV/r + RAL)
RADAR (DRV/r + RAL)
SPARTAN (ATV + RAL)
CCTG 589 (LPV/r + RAL)
A4001076 (ATV/r + MVC)
VEMAN (LPV/r + MVC)
MIDAS (DRV/r + MVC
LOREDA (LPV/r + 3TC)
ACTG 5142 (LPV/r + EFV)
ARV-experienced
KITE (LPV/r + RAL)
ATLAS (ATV/r + 3TC)
A5116 (LPV/r + EFV)
NEKA (LPV/r + NVP)
PRESENTED AT CROI 2013
SECOND LINE:
LPV/r +RAL vs. LPV/r + NRTIs after first-line virologic failure
Wk 48 primary endpoint
Stratified by clinical site, baseline HIV-1 RNA
(≤ or > 100,000 copies/mL)
LPV/r 400/100 mg BID +
RAL 400 mg BID
(n = 270)
HIV-infected pts with virologic failure on first-line regimen of 2
NRTIs + NNRTI
(N = 541)
LPV/r 400/100 mg BID +
2-3 NRTIs QD or BID
(n = 271)
Humphries A, et al. CROI 2013. Abstract 180LB.
PRESENTED AT CROI 2013
SECOND LINE:
Non-inferiority of LPV/r + RAL vs. LPV/r + NRTIs
Design:
Randomized, open-label study conducted at 38 sites in 15 countries
Similar high levels of virologic suppression with each strategy in primary mITT analysis
100
Subjects:
541 HIV1 positive adults (≥16 years) with virologic failure with first-line ART (NNRTI
+ 2N(t)RTIs) for ≥24 weeks
80
60
Treatment arms (1:1 randomization):
•
LPV/r + 2-2 N(t)RTIs (control)
•
LPV/r + RAL [N(t)RTI-sparing]
40
Primary objective:
Comparison of the antiviral efficacy of second-line ART regimens (% with plasma
HIV RNA <200 copies/mL after 48 weeks)
20
0
Humphries A, et al. CROI 2013. Abstract 180LB. Graphic used with permission.
Zheng Y, et al. CROI 2013. Abstract 558.
0 12 24
Wk
36
82.6
80.8
P = .59
LPV/RTV + RAL
LPV/RTV + 2-3 NRTIs
48
PRESENTED AT CROI 2013
ROCnRAL ANRS 157:
Switch to MVC+RAL in lipodystrophic patients with suppressed viral load
Key inclusion criteria:
HIV RNA < 50 copies/mL
CD
4 cell count nadir >100 cells/mm 3
Switched from suppressive
HAART
Clinical lipodystrophy
RAL 400 mg BID
+ MVC 300 mg BID
(n=44)
24 weeks
Primary endpoint
48 weeks
Primary endpoint
Proportion of patients with treatment failure at week 24 (ITT)
(Defined as either virological failure with 2 consecutive plasma HIV RNA >50 copies/mL or treatment discontinuation)
CROI 2013, Abstract #566
PRESENTED AT CROI 2013
ROCnRAL ANRS 157:
Switch to MVC+RAL in lipodystrophic patients with suppressed viral load
7 patients discontinued therapy
•
5 had virologic failure; 3 due to adverse events
•
3/5 failures had resistance to RAL (A. F121Y, Y143C, N155H
Premature discontinuation of study advised by DSMB
CROI 2013, Abstract #566
Maraviroc + Raltegravir Dual Therapy in Aviremic HIV +
Patients with Lipodystrophy: Virologic Failures and Resistance
Patient
1
2
3
4
5
W-4 screening Baseline cART prior entry
Duration of suppressed viremia (yrs)
CD4 count
(mm 3 )
Viral tropism
(on DNA)
HIV-1 viral load (c/mL)
TDF / FTC /
EFV
DRV/r
TDF / FTC /
DRV/r
TDF / FTC /
DRV/r
TDF / FTC /
EFV
9.5
7.5
9.8
3.6
6.6
477
832
893
601
954
CCR5
CCR5
CCR5
CCR5
CCR5
105
2973
598
8972
1810
1453
204
69
8070
27434
259
375
2820
Virological Failure
Time of failure
Drug concentrations plasma C min
(ng/mL)
Integrase mutation resistance
Viral tropism on
HIV-RNA
W4
W8
W12
W16
W18
W20
W24
RAL: 21
MVC: 13
RAL:
1960
MVC: 160
No mut. to RAL
RAL:
VT2I,
Y143C
CCR5
CXCR4
W16
W20
W12
W16
RAL: 56
MVC: 104
RAL: 121
MVC: 28
RAL:
VT2I,
N155H
RAL:
VT2I
CCR5
CCR5
W20
W22
RAL: 87
MVC: 105
RAL:
F121Y
CCR5
Katlama C, et al. Presented at CROI 2013; poster #566.
Maraviroc + Raltegravir Dual Therapy in Aviremic HIV +
Patients with Lipodystrophy: Serious AEs Leading to Discontinuation
Patient Characteristics Serious AE
Timing of
SAE
Patient 6
Patient 7 •
•
•
•
• CCR5 tropism
• cART: ABC/3TC/ATV
• Suppressed viremia: 6.2 yrs
• CD4 cell count: 715/mm 3
• HBsAg-, HBsAb-, HBcAb+
CCR5 tropism cARTL: TDF/FTC/RTV
Suppressed viremia: 5.6 years
CD4 cell count: 594/mm 3
HBV reactivation
AST/ALT > 20xN
(grade 4)
Related to lamivudine discontinuation
Cutaneous rash and diarrhea possibly related to raltegravir and maraviroc
Katlama C, et al. Presented at CROI 2013; poster #566.
W16
W4
Ongoing Studies
• ANRS 143 Study:
Study to determine whether the combination regimen of DRV/r and RAL is not inferior to the combination therapy involving the DRV/r and TDF-FTC combination in 800 adults infected with HIV-1 without a history of ARV treatment, for at least 96 weeks.
• MODERN Study:
– Phase III study (A4001095) comparing a CCR5 antagonist (maraviroc) with the combination regimen TDF-FTC (Truvada
®
) both taken in combination with DRV/r for 96 weeks, in 804 patients.
• LPV/r and lamivudine (3TC):
–
Comparison of the tolerability and efficacy of LPV/r taken with 3TC and LPV taken with two
NRTIs in 407 subjects with no history of ARV treatment for 96 weeks.
• HARNESS Study
– Phase IV study comparing the switch from a treatment with atazanavir 300 mg/ritonavir
100 mg QD combined with either raltegravir BID or tenofovir/emtricitabine QD in patients with an undetectable viral load under tri-therapy (120 patients).
ONGOING STUDIES OF PI-BASED
NRTI-SPARING REGIMENS
LPV/r + RAL
LPV/r + 3TC
2013
STUDY
EARNEST
(treatment failure)
GARDEL
(ARVnaïve)
N
400
205
2014
STUDY
SELECT
(treatment failure)
OLE
(simplification)
N
350
168
TOTAL
N
750
372
DRV/r + RAL
DRV/r + MVC
DRV + ETV
NEAT001
(ARVnaïve)
MODERN
(ARVnaïve)
GUSTA
(Switch)
PK
INROADS
Phase 2
(treatment failure)
400
402
165
15
54
400
402
165
15
54
ATV + RAL
N= dual therapy arm
HARNESS
(Switch)
60 60
CONCLUSIONS
•
Despite the numerous available ARV combinations, no treatment regimen is free of adverse effects.
•
Most PLHIV are now treated and will be so for most of their lives. The following should be taken into consideration :
–
–
Long-term toxicities
Existing co-morbidities
Cross toxicities
Drug interactions
•
Treatments should be individualized to take each patient ’ s circumstances into account. There is a need for NRTI-sparing treatments:
–
Patients who are intolerant of NRTIs
–
Resistance to NRTIs
Co-morbidities exacerbated by NRTIs
•
Studies are underway of PI/r combinations with raltegravir, maraviroc and 3TC and will be able to better support this practice in the future. For now, the preliminary results show that not all of these combinations are equivalent.
ACKNOWLEDGEMENTS
The Working Group on Innovative
Strategies for HIV Care