Methylene Blue and Catecholamine Nonresponsive
advertisement
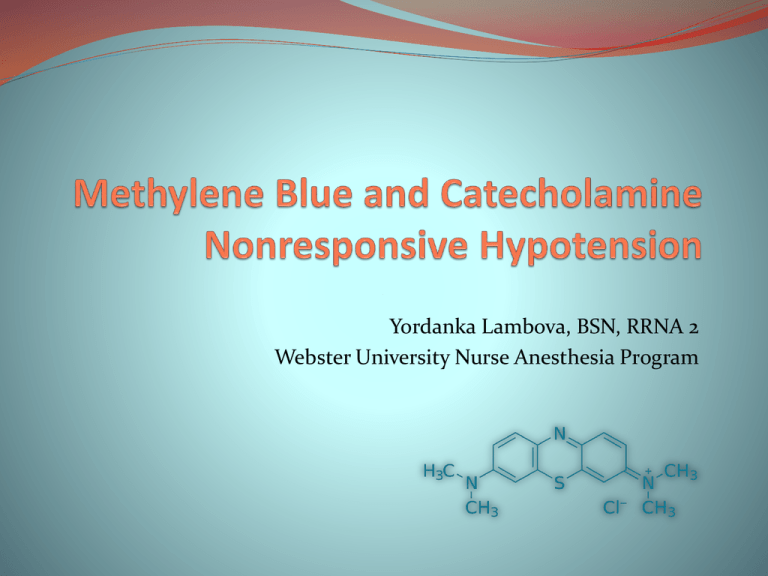
Yordanka Lambova, BSN, RRNA 2 Webster University Nurse Anesthesia Program Objectives Vasoplegic Syndrome Definition Risk Factors Pathophysiology Methylene Blue Pharmacology Application Side Effects Evidence Based Practice “Vasoplegic Syndrome”(VS) --Gomez (1994)—vasodilatory phenomenon refractory to high dose catecholamines in adult cardiac surgery1,2 Gomes W. J. et al.; J Thorac Cardiovasc Surg 1994;107:942-a-943-a Copyright ©1994 The American Association for Thoracic Surgery Vasoplegic Syndrome Observed in all age groups and clinical settings Sepsis Systemic inflammatory response syndrome (SIRS) Cardiac surgery Anaphylactic shock Cardiac Surgery Heparin and renin-angiotensin system (RAS) antagonists are the only medications considered risk factor for VS3 Incidence with cardiac surgery is ranging from 0.21 to 13%, up to 50% when patients on RAS antagonists4 Mortality rate is 16 to 27%5,6,7 Vasoplegic Syndrome Common Etiology Pathway Endothelial injury and release of vasodilatory inflammatory mediators Tumor necrosis factor alpha (TNF-α) Interferon gamma (IFN-γ) Interleukin-1 (IL-1) Atrial Natriuretic Peptide (ANP) Arginine-vasopressin system dysfunction and deficiency of vasopressin hormone KATP channel activation in the plasma membrane Inducible Nitric Oxide Synthase (iNOS) activation •Nitric oxide (NO) production from L- arginine is catalyzed by a family of NO synthases (NOS) •Endothelial NOS (eNOS) provides a basal release of NO to maintain smooth muscle vascular tone •iNOS in heart, lungs, and vascular smooth muscle cells is up regulated by the influence of proinflammatory cytokines and/or endotoxin •Large amount of NO is produced •Soluble guanylate cyclase (sGC) is released •Cyclic guanosine 3’-5’ monophosphate (cGMP) is generated •Smooth muscle cell cGMP-mediated vasodilation and decrease myocyte contractility—relaxation of myocardial and vascular smooth muscle What do I reach for with catecholamine nonresponsive hypotension? •Persistent hypotension •Tachycardia •Normal or increased cardiac output •Decreased systemic vascular resistance •Low filling pressure •Poor or no response to fluid resuscitation and vasopressors Methylene Blue (MB) Something Old, Something Blue Prepared by Caro in 1876 as a dye for textiles First fully synthetic drug used in medicine 1891 Paul Ehrlich identified the compound as an anti-malarial 1899 positive psychotropic effects observed (Potent, but reversible MAOI) 1933 used as an antidote to cyanide poisoning Beginning of the 20th century MB used in a wide variety of medical, hygienic, and microbiology compounds MB: Chemical Properties Heterocyclic aromatic chemical compound Chemical formula C16H18N3SCl Melting temperature 180 degrees Solubility in water 35.5 g/1 pH value—3 (10g/l H2O) Solid, odorless, dark green powder at room temperature Blue solution when dissolved in water or alcohol Three molecules of MB per molecule of water MB: Pharmacokinetics Oral absorption is between 53 to 97% Completely ionized at gastric pH Peak plasma concentration in 30-60 min Volume of distribution 20 ml/kg Plasma half-life 5-6 hrs Metabolism reduced in peripheral tissues to leucomethylene blue (65-85%) Does not bind to plasma proteins Eliminated in bile, feces, and urine as leucomethylene blue MB: Dosing in Humans Sepsis9,10 1-2 mg/kg/10-20 min IV bolus 0.25-1 mg/kg/hr for 6hrs IV continuous infusion Anaphylactic shock11 1.5-2 mg/kg IV bolus Hereditary methemoglobinemia Up to 300mg/day PO Ifosfamide encephalopathy 50 mg three times a day PO12 Vasodilation with hypotension5,6 1-2 mg/kg/10-20 min IV bolus VS8 1-2 mg/kg/10-20 min IV bolus 0.25-1 mg/kg/hr IV 48-72 hrs IV continuous infusion Surgery for septic endocarditis13, Cardiopulmonary bypass (CPB)5,11,14 2 mg/kg IV bolus prior to CPB 0.5 mg/kg/hr after bolus for 30 min after CPB MB: Dose Related Toxicity Human Studies Studies Dose mg/kg Toxic Manifestations14,15 Dose mg/kg Animal Toxic Manifestations 2-4 Hemolytic anemia, skin desquamation in infants 5-50 Rat16 Neuronal apoptosis 1250 mg/kg LD50 7 Nausea, vomiting, abdominal pain, chest pain, fever, hemolysis 3500 Mouse 7.5 Hyperpyrexia, confusion 40 Sheep17 20 Hypotension 10-20 Dog18 80 Bluish discoloration of skin (similar to cyanosis) Hypotension, decreased SVR, renal blood flow; pulmonary hypertension MB: Contraindications Glucose-6-phosphate dehydrogenase deficiency-may precipitate hemolytic anemia Renal impairment— acceptable if on dialysis Intrathecal and subcutaneous injection Hypersensitivity and allergy to MB Dapsone- forms hydroxylamine causing hemolysis FDA recommendation-MB should not be given to patients taking serotonergic drugs. However, there are some conditions that may be life-threatening or require urgent treatment with MB such as methemoglobinemia, ifosfamide-induced encephalopathy, or cyanide poisoning. MB: Mechanism of Action in VS Direct inhibitory effect on NOS19,20 Blocks accumulation of cGMP by inhibiting the enzyme guanylate cyclase19 Blocks the activity of NO-dependent guanylate cyclase via oxidation of the active haemo center or by inactivation of its haemo center.19,20 More specific and potent inhibitor of NOS than guanylyl cyclase– NO-donating compounds in the presence of MB can still partially activate c-GMP signaling pathways21,22 Effects due to NO inhibition MB restores vascular reactivity to endogenous catecholamines in the setting of excessive NO production23 Not a vasoconstrictor, rather it acts as a liberator of cAMP, thus allowing norepinephrine to exert its vasoconstrictive effect24 MB: Hepatic Failure Schenk et al (2000)25 Case Series (n=10) of hepatic cirrhosis and hepatopulmonary syndrome patients Reports improvement of hypoxemia and hyperdynamic circulation evidenced by significant increase in PaO2, SVR; and decrease in MPAP, PVR, CO MB 3 mg/kg IV over 15 minutes No significant side effects noted Kalambokis (2005)26 Investigational study (n=20, 10 experimental group, 10 placebo group) on cirrhosis and ascites patients MB 3 mg/kg IV No change in MAP, HR, CO, SVR; plasma renin, aldosterone, antidiuretic hormone, urea, Cr, Na, GFR, Serum NO, and urinary Na decreased 4 hrs, but returned to basal levels in 8 hrs Almeida et al (2007)27 Case report (n=1) on use of MB in hepatopulmonary syndrome Large right to left intrapulmonary shunt and subsequent improvement of vascular tone and hyperdynamic circulation at the cost of worsening hypoxemia MB: Renal Failure Peer (2001)28 Investigational study (n=41, 18 HD/HoTN, 18 HD/no HoTN, 5 healthy controls) MB IV bolus 1 mg/kg followed by infusion of 0.1 mg/kg for 210 minutes until completion of HD, bolus dose only on days without HD Results HD/HoTN—completely prevented HoTN during HD, increased BP on non HD days, blood NO measurements higher than other groups HD/no HoTN—increase of BP during first hour of HD, and 90 minutes on non HD days. Healthy controls—no significant change in BP MB: Sepsis Daemen-Gubbels et al (1995)29 Prospective observational (n=9) MB 2 mg/kg/20 min Increase in MAP, MPAP, LVSWI, RVSWI (p<0.01); increase in oxygen delivery and uptake index (p<0.05) Mortality: 89% Kirov et al (2001)9 Prospective, randomized, placebo controlled study (n=20, 10 MB treatment and 10 placebo isotonic saline) MB 2 mg/kg/20min, 0.25 mg/kg/hr at 2hrs, 0.5 mg/kg/hr at 3hrs, 1 mg/kg/hr at 4 hrs, 2 mg/kg/hr at 5hrs for 1 hr Improvement in hemodynamics and decrease in vasoconstrictors and inotropes (p<0.05) Mortality: 5 patients vs. 7 in placebo group Memmis et al (2002)30 prospective, randomized, double blind, placebo controlled (n=30, 15 MB and 15 placebo isotonic saline) MB 0.5 mg/kg/hg 6 hrs MB group increase in MAP (p<0.001) Mortality: 27% in both cohorts MB: Cardiac Surgery Andrade et al.(1996)31 Cohort (n=6), with and without cardiopulmonary bypass (CBP) Criteria—tachycardia, oliguria, refractory hypoperfusion to high dose catecholamine MB 1.5 mg/kg/1 hr Results: restoration of blood pressure; pre-MB vs. post-MB SVR=868 vs. 1693 dyne/s/cm5; no adverse effect on CO or PVR 2b level of evidence—small cohort, limited data Leyh et al. (2003)5 Cohort (n=54) out of 1111 cardiac surgery patients in 12 months Criteria –CO 4l/min, SVR<600 dyne/s/cm5, norepinephrine 0.5 mcg/kg/min MB 2mg/kg/20 min Results (0 hr vs. 1hr vs. 6hrs vs. 12 hrs): MAP 68 vs. 72 vs. 73, (p<0.02) CO 7.6 vs. 6.5 vs. 5.8, (p<0.001) SVR 547 vs. 766 vs. 876, (p<0.001) 4/54 (7.4%) no response to treatment 3/54 (5.6%) mortality rate—2/4 nonresponders) 2b level of evidence—wide range of cardiac surgical procedures, no control group, 76% male patients MB: Cardiac Surgery Levin et al. (2004)6 Cohort progressing to PRCT (n=56) out of 638 cardiac surgery patients in 5 months Criteria—MAP<50 mmHg, CVP<5 mmHg, PCWP<10mmHg, CI=2.5 l/min/m3, SVR<800 dyne/s/cm5, vasopressor requirement MB 1.5 mg/kg/1 hr vs. placebo Results: MB VS vs. Placebo VS Duration of VS: less than 2 hrs vs. up to 48 hrs (p<0.0007) Vasopressor requirement: at 2 hrs (p<0.002); at 3, 6, 12, 24 hrs postop (p<0.00) Renal failure, respiratory failure, myopathy: 2 vs. 8, (p<0.03) Sepsis and MODS: 0 vs. 7, (p<0.005) Mortality: 0% vs. 21.4%, (p<0.01) 1b level of evidence—small numbers progressing to RCT with patients from 4 centers, questionable random assignment due to uneven distribution of patients in different hospitals (2,9,14, 31) MB: Cardiac Surgery Ozar et al. (2005)7 PRCT (n=100), high VS risk patients divided equally in MB and placebo group Criteria—MAP<50 mmHg, CVP<5 mmHg, PCWP<10mmHg, CI=2.5 l/min/m3, SVR<800 dyne/s/cm5, norepinephrine requirement 0.5 mcg/kg/min MB 2 mg/kg/30 min 1 hr preoperatively Results: MB vs. Placebo Incidence of VS: 0 vs. 13 (p<0.001) Progress of VS: 6 placebo patients had refractory to norepinephrine VS (p<0.001) 4/6 resolved in up to 8 hours, 2/6 died of MSOF SVR on CBP significantly higher in MB group (p<0.001) Norepinephrine requirement: To keep MAP on CBP >45: 4% vs. 82 % Required NE 0.5 mcg/kg/min (p<0.001) Fluid Requirement on CBP: Crystalloid (p=0.024) Colloid (p=0.027) RBC (p<0.001) Length of stay: ICU: 1.2 vs. 2.1, (p<0.001) Hospital 6.1 vs. 8.4, (p<0.001) 1b level of evidence Conclusion MB is a novel therapeutic option for patients with VS Despite the abundance of case reports on the use of MB as a rescue drug there are limited number of cohort/RCTs evaluating the use of the drug Further large studies should be performed before MB can be recommended as a first line therapy. References: 1. Gomes et al. Vasoplegic syndrome: a new dilemma. J Thorac Cardiovasc Surg 1194. 2. Gomes et al. Vasoplegic syndrome after off-pump coronary artery bypass surgery. Eur J Cardiothorac Surg 2003. 3. Ulusoy HB, Gul H, Seyrek M, Yildiz O, Ulku C, Yildirim V, et al. The concentration-dependent contractile effect of methylene blue in the human internal mammary artery: a quantitative approach to its use in the vasoplegic syndrome. J Cardiothorac Vasc Anesth. 2008; 22(4): 560-4. 4. Mekontso-Dessap A, Houel R, Soustelle C, Kirsch M, Thebert D, Loisance DY. Risk factors for postcardiopulmonary bypass vasoplegia in patients with preserved left ventricular function. Ann Thorac Surg 2001;71:1428-1432. 5. Leyh RG, Kofidis T, Strüber M, Fischer S, Knobloch K, Wachsmann B, et al. Methylene blue: the drug of choice for catecholamine-refractory vasoplegia after cardiopulmonary bypass? J Thorac Cardiovasc Surg. 2003; 125(6): 1426-31. 6. Levin RL, Degrange MA, Bruno GF, Del Mazo CD, Taborda DJ, Griotti JJ, et al. Methylene blue reduces mortality and morbidity in vasoplegic patients after cardiac surgery. Ann Thorac Surg. 2004; 77(2): 496-9. 7. Ozal E, Kuralay E, Yildirim V, Kilic S, Bolcal C, Kücükarslan N, et al. Preoperative methylene blue administration in patients at high risk for vasoplegic syndrome during cardiac surgery. Ann Thorac Surg. 2005; 79(5): 1615-9. 8. 10. Evora PR, Levin RL. Methylene blue as drug of choice for cate- cholamine-refractory vasoplegia after cardiopulmonary bypass. J Thorac Cardiovasc Surg 2004; 127: 895-896 . 9. Kirov MY, Evgenov OV, Evgenov NV, et al. Infusion of methylene blue in human septic shock: a pilot, randomised, controlled study. Crit Care Med 2001; 29: 1860-1867 10. Kwok ES, Howes D. Use of methylene blue in sepsis: a systematic review. J Intensive Care Med 2006; 21: 359363 11. Evora PR, Oliveira Neto AM, Duarte NM, Vicente WV. Methylene blue as treatment for contrast mediuminduced anaphylaxis. J Postgrad Med 2002; 48: 327-328. 12. Pelgrims J, De Vos F, Van den Brande J, Schrijvers D, Prové A, Vermorken JB. Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: report of 12 cases and a review of the literature. Br J Cancer 2000; 82: 291-294. 13. Grayling M, Deakin CD. Methylene blue during cardiopulmonary bypass to treat refractory hypotension in septic endocarditis. J Thorac Cardiovasc Surg 2003; 125: 426-427 14. Maslow AD, Stearns G, Butala P, Schwartz CS, Gough J, Singh AK. The hemodynamic effects of methylene blue when administered at the onset of cardiopulmonary bypass. Anesth Analg 2006; 103: 2-8 . 15. Mathew S, Linhartova L, Raghuraman G. Hyperpyrexia and pro- longed postoperative disorientation following methylene blue infusion during parathyroidectomy. Anaesthesia 2006; 61: 580- 583. 16. Vutskits L, Briner A, Klauser P, et al. Adverse effects of methylene blue on central nervous system. Anesthesiology 2008; 108: 684- 692. 17. Burrows GE. Methylene blue: effects and disposition in sheep. J Vet Pharmacol Ther 1984; 7: 225-231 18. Zhang H, Rogiers P, Preiser JC, et al. Effects of methylene blue on oxygen availability and regional blood flow during endotoxic shock. Crit Care Med 1995; 23: 1711-1721 19. Mayer B, Brunner F, Schmidt K. Inhibition of nitric oxide syn- thesis by methylene blue. Biochem Pharmacol 1993; 45: 367- 374. 20. Mayer B, Brunner F, Schmidt K. Novel actions of methylene blue. Eur Heart J 1993; 14; Suppl I: 22-26. 21. Martin W,Villani GM, Jothianandan D, Furchgott RF. Selective blockade of endothelium-dependent and glyceryl trinitrate- induced relaxation by hemoglobin and by methylene blue in rabbit aorta. J Pharmacol Exp Ther 1985; 232: 708-716. 22. Tsai SC, Adamik R, Manganiello VC, Vaughan M. Regulation of activity of purified guanylate cyclase from liver that is unresponsive to nitric oxide. Biochem J 1983; 215: 447-455 23. Schneider F, Luton PH, Hasselmann M, et al. Methylene blue increases systemic vascular resistance in human septic shock. Intensive Care Med 1992; 9: 309-311. 24. Schneider F, Bucher B, Schott C, Andre A, Julou-Schaeffer G, Stoclet JC. Effect of bacterial lipopolysaccharide on function of rat small femoral arteries. Am J Physiol 1994; 266: H191-198 25. Schenk P, Madl C, Rezaie-Majd S, Lehr S, Muller C. Methylene blue improves the hepatopulmonary syndrome. Ann Inten Med 2000; 133: 701-706. 26. Kalambokis G, Economou M, Fotopouslos A, Bokharhii JA, Christos P, Paraskevi K, Kontanstinos P, Katsaraki A, Tsinas EV. Effects of nitric oxide inhibition by methylene blue in cirrhotic patients with ascites. Dig Liver Dis 2003; 50:1771-1777. 27. Almeida JA, Riordan SM, Liu J, Galhenage S, Kim R, Bihari D, Wegner EA, Cranney GB, Thomas PS. Deleterious effects of nitric oxide inhibition in chronic hepatopulmonary syndrome. Eur J Gastroenterol Hepatol.2007;19:34-134.6. 28. Peer G, Itzhakov E, Wollman Y, Chernihovsky T, Grosskopf I, Segev D, Silverberg D, Blum M, Schwartz D, Iaina A. Methylene blue, a nitric oxide inhibitor, prevents haemodialysis hypotension. Nephrol Dial Transplant 2001; 16:14361441. 29. Daemen-Gubbels CR, Groeneveld PH, Groeneveld AB, van Kamp GJ, Bronsveld W, Thijs LG. Methylene blue increases myocardial function in septic shock. Crit Care Med 1995;23:1363–70. 30. Memis D, Karamanlioglu B, Yuksel M, Gemlik I, Pamukcu Z. The influence of methylene blue infusion on cytokine levels during severe sepsis. Anaesth Intensive Care 2002;30:755–62. 31. Andrade JCS, Batista Filho ML, Evora PRB, Tavares JR, Buffolo E, Ribeiro EE, Silva LA, Teles CA, Petrizzo A, Barata F, Vitor V, Duprat R. Methylene blue administration in the treatment of the vasoplegic syndrome after cardiac surgery. Revista Brasileira de Cirurgia Cardiovascular (Rev Bras Cir Cardiovasc) 1996;11:107-114.