Reorganization of understory diversity over three decades in an old
advertisement
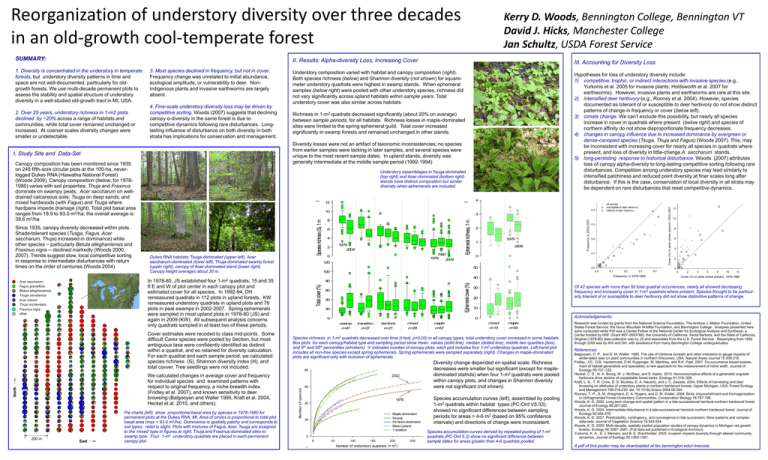
Reorganization of understory diversity over three decades in an old-growth cool-temperate forest SUMMARY: 4. Fine-scale understory diversity loss may be driven by competitive sorting. Woods (2007) suggests that declining canopy α-diversity in the same forest is due to competitive dynamics following rare disturbances. Longlasting influence of disturbance on both diversity in both strata has implications for conservation and management. Canopy composition has been monitored since 1935 on 248 fifth-acre circular plots at the 100-ha, neverlogged Dukes RNA (Hiawatha National Forest) (Woods 2009). Canopy composition (below, for 19781980) varies with soil properties: Thuja and Fraxinus dominate on swampy peats; Acer saccharum on welldrained calcareous soils; Tsuga on deep sands; and mixed hardwoods (with Fagus) and Tsuga where hardpans impede drainage (right). Total plot basal area ranges from 18.9 to 93.0 m2/ha; the overall average is 39.6 m2/ha. North 12 2 2 Pie charts (left) show proportional basal area by species in 1978-1980 for permanent plots at the Dukes RNA, MI. Area of circles is proportional to total plot basal area (max = 93.0 m2/ha). Dominance is spatially patchy and corresponds to soil types; relief is slight. Plots with mixtures of Fagus, Acer, Tsuga are assigned to the ‘mixed’ type in figures at right; Thuja and Fraxinus-dominated sites to swamp type. Four 1-m2 understory quadrats are placed in each permanent canopy plot 6 4 1979 2004 1992 1979 2004 0 120 Ephemeral richness, 1 m 8 2 4 3 2 1979 1 2009 0 50 100 40 80 Ephemeral cover (%) Dukes RNA habitats: Tsuga-dominated (upper left), Acer saccharum-dominated (lower left), Thuja-dominated swamp forest (upper right), canopy of Acer-dominated stand (lower right). Canopy height averages about 30 m. We calculated changes in average cover and frequency for individual species and examined patterns with respect to original frequency, a niche-breadth index (Fridley et al. 2007), and known sensitivity to deer browsing (Balgooyen and Waller 1995, Kraft et al. 2004, Heckel et al. 2010, and others). East Richness in 1-m2 quadrats decreased significantly (about 20% on average) between sample periods, for all habitats. Richness losses in maple-dominated sites were limited to the spring ephemeral guild. Total cover increased significantly in swamp forests and remained unchanged in other stands. 10 Cover estimates were recoded to class mid-points. Some difficult Carex species were pooled by Section, but most ambiguous taxa were confidently identified as distinct within a quadrat, and so retained for diversity calculation. For each quadrat and each sample period, we calculated species richness (S), Shannon diversity index (H), and total covver. Tree seedlings were not included. 200 m Hypotheses for loss of understory diversity include: 1) competitive, trophic, or indirect interactions with invasive species (e.g., Yurkonis et al. 2005 for invasive plants; Holdsworth et al. 2007 for earthworms). However, invasive plants and earthworms are rare at this site. 2) intensified deer herbivory (e.g., Rooney et al. 2004). However, species documented as tolerant of or susceptible to deer herbivory do not show distinct patterns of change in frequency or cover (below left). 3) cimate change. We can’t exclude this possibility, but nearly all species increase in cover in quadrats where present (below right) and species of northern affinity do not show disproportionate frequency decreases. 4) changes in canopy influence due to increased dominance by evergreen or dense-canopied species (Tsuga, Thuja and Fagus) (Woods 2007). This, may be inconsistent with increasing cover for nearly all species in quadrats where present, and loss of diversity in little-change A. saccharum stands. 5) long-persisting response to historical disturbance. Woods (2007) attributes loss of canopy alpha-diversity to long-lasting competitive sorting following rare disturbances. Competition among understory species may lead similarly to intensified patchiness and reduced point diversity at finer scales long after disturbance. If this is the case, conservation of local diversity in all strata may be dependent on rare disturbances that reset competitive dynamics. Understory assemblages in Tsuga-dominated (top right) and Acer-dominated (bottom right) stands have distinct composition but similar diversity when ephemerals are included. In 1978-80, JS established four 1-m2 quadrats, 15 and 35 ft E and W of plot center in each canopy plot and estimated cover for all species. In 1992-94, DH remeasured quadrats in 112 plots in upland forests. KW remeasured understory quadrats in upland plots and 76 plots in peat swamps in 2002-2007. Spring ephemerals were sampled in most upland plots in 1978-80 (JS) and again in 2009 (KW). All subsequent analysis concerns only quadrats sampled in at least two of these periods. Acer saccharum Fagus grandifolia Betula alleghaniensis Tsuga canadensis Acer rubrum Thuja occidentalis Fraxinus nigra other Understory composition varied with habitat and canopy composition (right). Both species richness (below) and Shannon diversity (not shown) for squaremeter understory quadrats were highest in swamp stands. When ephemeral samples (below right) were pooled with other understory species, richness did not vary significantly across upland habitats within sample years. Total understory cover was also similar across habitats. Diversity losses were not an artifact of taxonomic inconsistencies; no species from earlier samples were lacking in later samples, and several species were unique to the most recent sample dates. In upland stands, diversity was generally intermediate at the middle sample period (1992-1994) I. Study Site and Data-Set Since 1935, canopy diversity decreased within plots. Shade-tolerant species (Tusga, Fagus, Acer saccharum, Thuja) increased in dominance) while other species – particularly Betula alleghaniensis and Fraxinus nigra -- declined markedly (Woods 2000, 2007). Trends suggest slow, local competitive sorting in response to intermediate disturbances with return times on the order of centuries (Woods 2004) III. Accounting for Diversity Loss Species richness (S), 1 m 2. Over 25 years, understory richness in 1-m2 plots declined by ~20% across a range of habitats and communities, while total cover remained unchanged or increased. At coarser scales diversity changes were smaller or undetectable. 3. Most species declined in frequency, but not in cover. Frequency change was unrelated to initial abundance, ecological amplitude, or vulnerability to deer. Nonindigenous plants and invasive earthworms are largely absent. II. Results: Alpha-diversity Loss, Increasing Cover Total cover (%) 1. Diversity is concentrated in the understory in temperate forests, but understory diversity patterns in time and space are not well-documented, particularly for oldgrowth forests. We use multi-decade permanent plots to assess the stability and spatial structure of understory diversity in a well-studied old-growth tract in MI, USA. Kerry D. Woods, Bennington College, Bennington VT David J. Hicks, Manchester College Jan Schultz, USDA Forest Service 60 40 20 0 Of 42 species with more than 50 total quadrat occurrences, nearly all showed decreasing frequency and increasing cover in 1-m2 quadrats where present. Species thought to be particularly tolerant of or susceptible to deer herbivory did not show distinctive patterns of change. 30 20 10 Acknowledgements: 0 swamp n=41 transition n=27 hemlock n=21 mixed n=29 maple n=67 mixed n=15 maple n=55 Species richness in 1-m2 quadrats decreased over time (t-test, p<0.05) in all canopy types; total understory cover increased in some habitats. Box plots for each canopy/habitat type and sampling period show mean values (solid line), median (dotted line), middle two quartiles (box), and 5th and 95th percentiles (whiskers) ‘n’ indicates number of canopy plots; each plot includes four 1-m2 understory quadrats. Left-hand plot includes all non-tree species except spring ephemerals. Spring ephemerals were sampled separately (right). Changes in maple-dominated plots are significant only with inclusion of ephemerals. Diversity change depended on spatial scale. Richness decreases were smaller but significant (except for mapledominated statnds) when four 1-m2 quadrats were pooled within canopy plots, and changes in Shannon diversity were not significant (not shown). Species accumulation curves (left), assembled by pooling 1-m2 quadrats within habitat types (PC-Ord V5.33), showed no significant differences between sampling periods for areas > 4-6 m2 (based on 95% confidence intervals) and directions of change were inconsistent. Species accumulation curves derived by repeated pooling of 1-m2 quadrats (PC-Ord 5.3) show no significant difference between sample dates for areas greater than 4-6 quadrats pooled. Research was funded by grants from the National Science Foundation, The Andrew J. Mellon Foundation, United States Forest Service, the Huron Mountain Wildlife Foundation, and Bennington College. Analyses presented here were conducted while KW was a Center Fellow at the National Center for Ecological Analysis and Synthesis, a Center funded by NSF (Grant #EF-0553768), the University of California, Santa Barbara, and the State of California. Original (1978-80) data-collection was by JS and associates from the U.S. Forest Service. Resampling from 1992 through 2009 was by KW and DH, with assistance from many Bennington College undergraduates. References: Balgooyen, C. P., and D. M. Waller. 1995. The use of Clintonia borealis and other indicators to gauge impacts of white-tailed deer on plant communities in northern Wisconsin, USA. Natural Areas Journal 15:308-318. Fridley, J.D., D.B. Vandermast, D.M. Kuppinger, M. Manthey, and R.K. Peet. 2007. Co-occurrence based assessment of habitat generalists and specialists: a new approach for the measurement of niche width. Journal of Ecology 95:707–722. Heckel, C. D., N. A. Bourg, W. J. McShea, and S. Kalisz. 2010. Nonconsumptive effects of a generalist ungulate herbivore drive decline of unpalatable forest herbs. Ecology 91:319–326. Kraft, L. S., T. R. Crow, D. S. Buckley, E. A. Nauertz, and J. C. Zasada. 2004. Effects of harvesting and deer browsing on attributes of understory plants in northern hardwood forests, Upper Michigan, USA. Forest Ecology and Management 199:219-230. doi: 10.1016/j.foreco.2004.05.044. Rooney, T. P., S. M. Wiegmann, D. A. Rogers, and D. M. Waller. 2004. Biotic Impoverishment and Homogenization in Unfragmented Forest Understory Communities. Conservation Biology 18:787-798. Woods, K. D. 2000. Long-term change and spatial pattern in a late-successional hemlock-northern hardwood forest. Journal of Ecology 88:267-282. Woods, K. D. 2004. Intermediate disturbance in a late-successional hemlock-northern hardwood forest. Journal of Ecology 92:464-476. Woods, K. D. 2007. Predictability, contingency, and convergence in late succession: Slow systems and complex data-sets. Journal of Vegetation Science 18:543-554. Woods, K. D. 2009. Multi-decade, spatially explicit population studies of canopy dynamics in Michigan old-growth forests. Ecology 90:3587–3587. (Full data-set published in Ecological Archives) Yurkonis, K. A., S. J. Meiners, and B. E. Wachholder. 2005. Invasion impacts diversity through altered community dynamics. Journal of Ecology 93:1053-1061. A pdf of this poster may be downloaded at fac.bennington.edu/~kwoods







